coord chem
to find oxidation states in such complexes we need to use the most stable state of metals right ? . here for example Cr will be in 3+ (most stable) , consequently Cr complex get 3- charge and Co complex get 3+ and therefore Co3+
however if i start with Co2+ i get Cr2+ which is wrong since Co2+ is stable but Cr2+ isnt as stable as Cr3+
correct logic ?
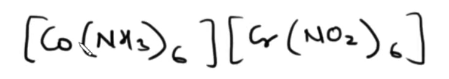
5 Replies
@Dexter
Note for OP
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.Yea sound logic. But this is an irl thing right. So with ioc logic can only get you so far. If in irl it is different then it is what it is
it isnt diff
+solved @iTeachChem
Post locked and archived successfully!
Archived by
<@741159941934415883> (741159941934415883)
Time
<t:1761809769:R>
Solved by
<@1035556259417571408> (1035556259417571408)