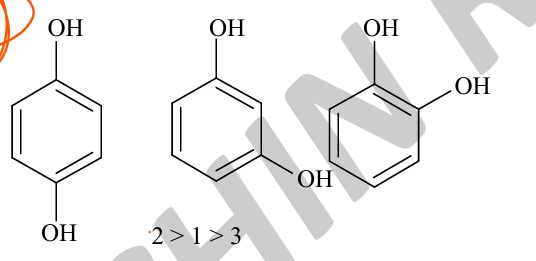
Acidity order
⚗️Chemistry
if we go by mesomeric effects:
OH shows +M, -I. Therefore at ortho and para positions the +M effect will destabilize the excess electron density formed at those pos.
At meta it shows -I only, therefore increasing stability.
if we go by H-Bonding
At para, inter molecular H bonding possible
At ortho intra is possible
toh us hisaa se Para > ortho > meta
OH shows +M, -I. Therefore at ortho and para positions the +M effect will destabilize the excess electron density formed at those pos.
At meta it shows -I only, therefore increasing stability.
if we go by H-Bonding
At para, inter molecular H bonding possible
At ortho intra is possible
toh us hisaa se Para > ortho > meta