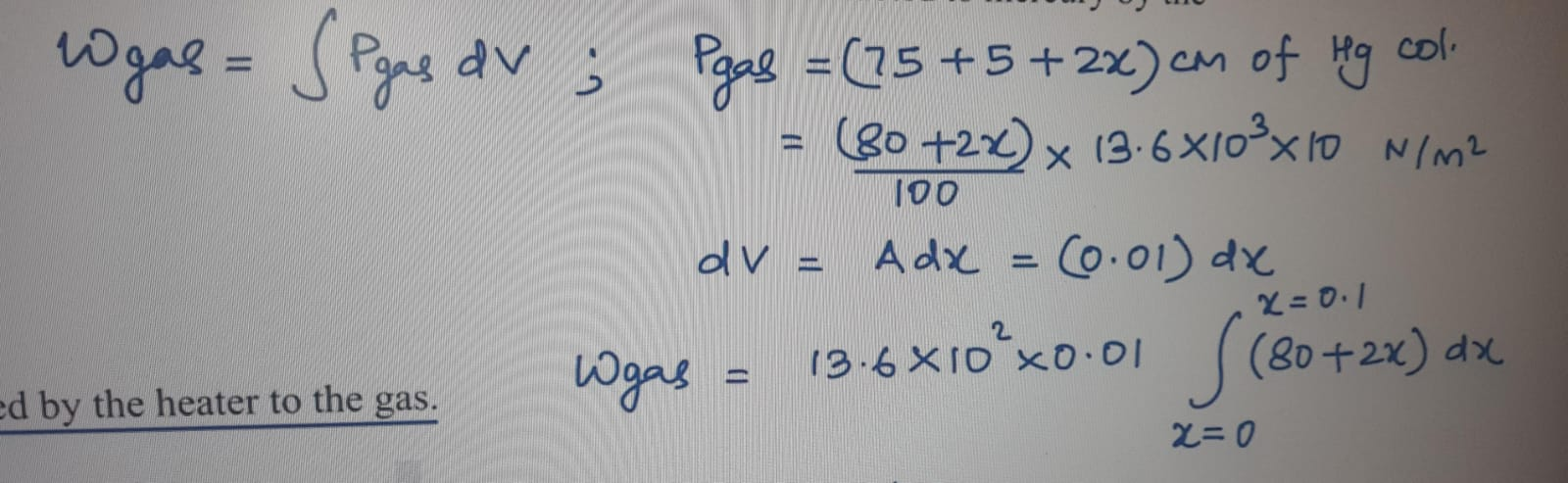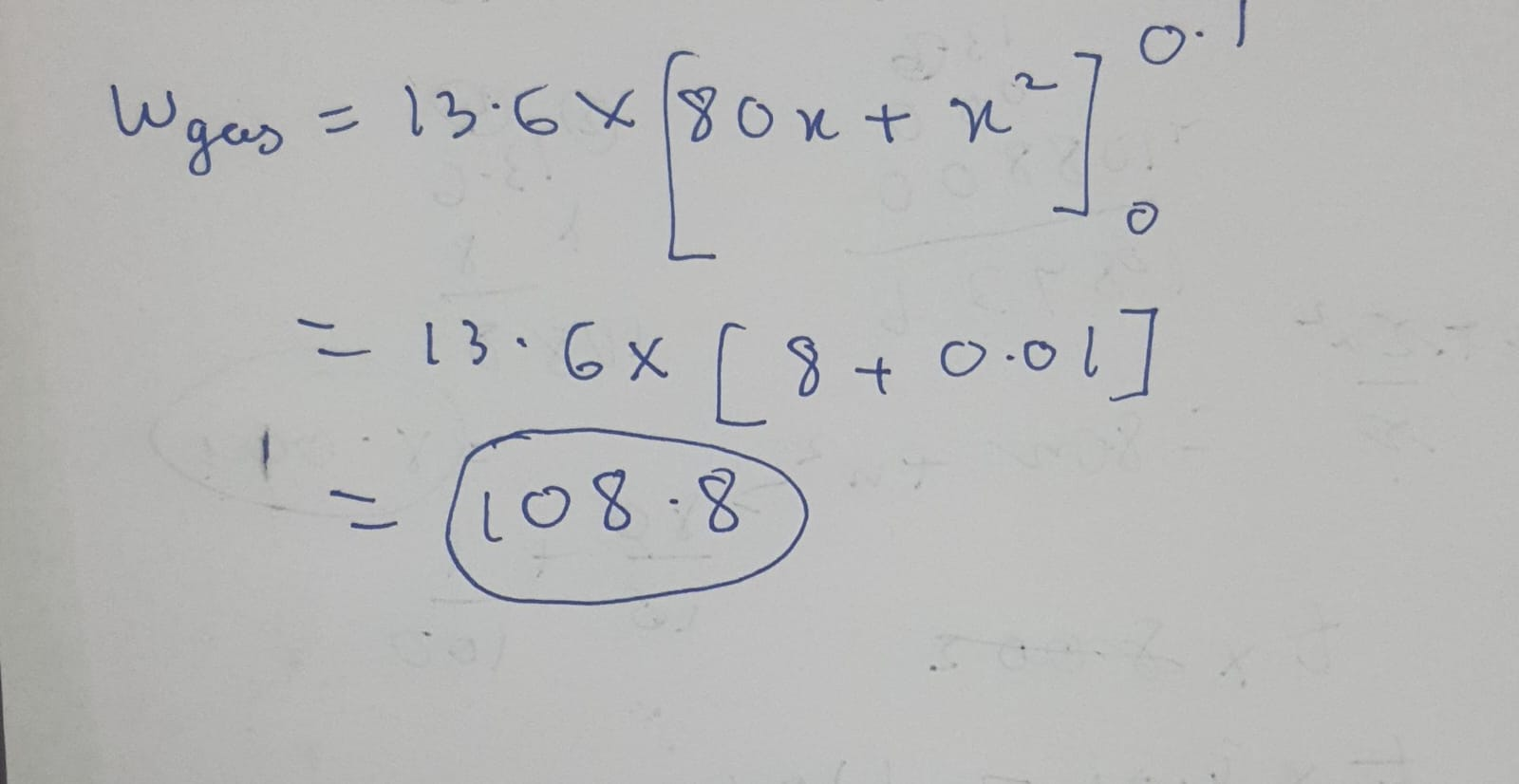14 Replies
@Gyro Gearloose
Note for OP
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.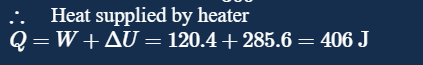
Sir I feel like my method is correct and I have also rechecked my calculations..so I honestly don't know :(
iteachchem
Transcription requested by Check
Hey Percy, so this difference that's coming out. Why do you think it's just mathematically number? Kewala, what do you think?
aapne work done ka calc dala hai, dU is the same?
Why have you integrated for work done and not used p delta V formula btw?
Since it is not mentioned that the process is isobaric, I decided to integrate because pressure varies with x.
na it says open to the atmosphere na
so we can assume an irreversible process
so pf = 80-2l, vf = 20+l, vi = 20, pi = 75+5, this tracks?
from this you get l = 10 cm
(which gives you the samework done i.e. 108.8 xD)
understood sir
But in the answer key it is given work done is 120.4 J....does that mean this answer is incorrect
must be
will wait for other physics folks to check
what did you get dU to be? moles you can get from PV/RT right? That x 3/2 R dT
Sir I got delta(U) as 285.6..yes sir I substituted the value of nR from nR = PV/T
+fsolved
Post locked and archived successfully!
Archived by
<@1035556259417571408> (1035556259417571408)
Time
<t:1748655067:R>