24 Replies
@Dexter
Note for OP
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.pv nrt shyd lagega
let me try
AAYEINNNN
IDHAR PVNRT
sab jagah a sakta hai pvnrt
aap ruko jara
Gas Law 🛐 🛐 🛐
kyunki stopper se ik extra pressure lageaga isiliye mere hisab se pv nrt ayega
temp ka del value mil jayega shyd
Bua ji supremacy
aise hi karna tha
mil gya kaise karna hai>
noice
mai jara kaam karne chli gyi thi
PYQ hai and truly majedaar
iteachchem
Transcription requested by Milky bar
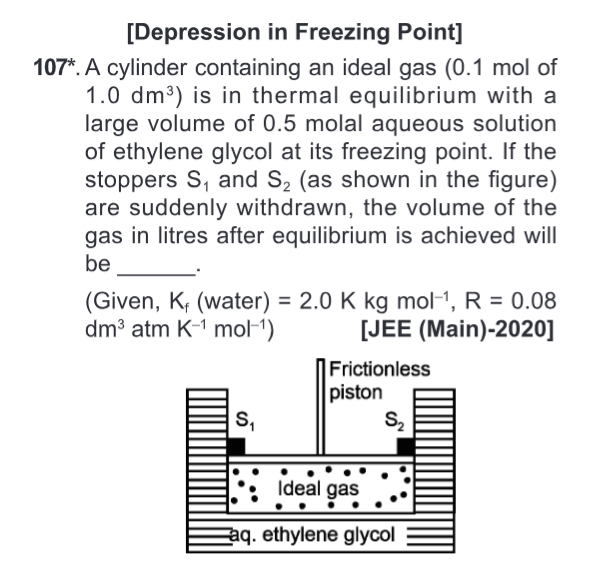
This is a very interesting question, this type of question कोई चांस नहीं जाएंगे मैं आएंगे अभी, but great question, because it involves ideas from depression and also ideal gas equation as Sir Frenad has correctly pointed out. What is Sir Frenad's new name now? Milky bar, so you get temperature and you find out what is the pressure, so you have P1 and V1's idea, after that you have to find out P2 and V2, P2 is known because if it is frictionless, then you find V2 that's it.
oooooooo nice detail
i wonder isme T variation aata to aur thoda diffuclt ho jaata
point tab aur bhi majedar
ah yes
which website is this?
Also, how will we find P2?
Oh I see
Mazedaar ques tha
Module ka pdf hai
bro we done here?
is this solved ? or u still dont understand
Yea no done done
+solved @Sephrina
Post locked and archived successfully!
Archived by
<@964432960197632059> (964432960197632059)
Time
<t:1748244329:R>
Solved by
<@888280831863451688> (888280831863451688)