16 Replies
@Dexter
Note for OP
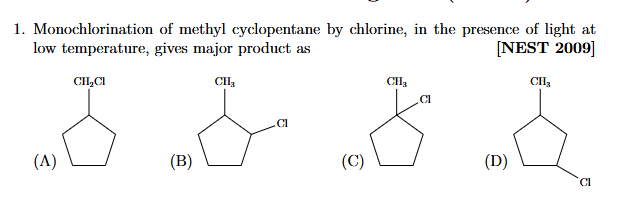
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.can we judge the radical stablility by alpha hydrogen ?
Yes you can
Hyperconjugation does act in radicals
oh
in this case if no 3 hona chahye answer ?
i dont have the answer key i am not sure
There's one catch here
"Low temperature"
oh
Think about kinetic and thermodynamic products
You'll get a bad mixture in this case to be very frank
was discussed very little in my class
But just for the sake of what the examiner is trying to ask, I think the answer should be A
Ohh I see
I can share a small article
You can read that up dw
@Varun_Arora i googled it answer is C sir
Ohh I see
Is the "low temperature" thing mentioned in that question as well
?
yess
low temp we take kinetic stable product ?
At low temperature, kinetic product prevails as the activation energy barrier is not met for the thermodynamically more stable product
https://chemistry.stackexchange.com/questions/59881/thermodynamic-vs-kinetic-reaction-control-with-radical-substitution
You can read this for a more detailed discussion
I think this thing is an overkill for an exam like NEST and they just mentioned the low temperature not considering all this
Nvm
Both kinetic and thermodynamic control are leading to the same product.
My bad
Sorry for confusing you on this
You'll understand what I mean when you read the first answer on this
oh
+solved @Varun_Arora
Post locked and archived successfully!
Archived by
<@905482015393062912> (905482015393062912)
Time
<t:1748675274:R>
Solved by
<@984016629119713290> (984016629119713290)