17 Replies
@Dexter
Note for OP
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.lol put topic in q please
in doubt
if it were a strong acid you would dilute by 10 times, right?
sorry sorry
why are you doing hydrolysis here?
weak acid , strong base
conjugate one
haan but why
wont the strong base react ?
this will happen if there is only A- in the solution and no H+
H+ hai toh
oohh
this is the q right
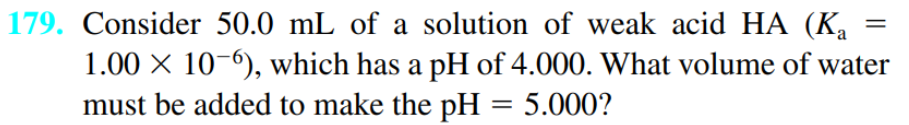
ys
gotta be careful about what eqn to use when :)
here, you are only diluting
yeah i will retry this without the unnecessary hydrolysis
yp got it
from the first setup you should be able to get initial molarity and hence moles of the acid
then you have new ph, moles, find new volume :) bas (cos you have ka too!)
yp got it now thnks sir
+solved @iTeachChem
Post locked and archived successfully!
Archived by
<@888280831863451688> (888280831863451688)
Time
<t:1749044481:R>
Solved by
<@1035556259417571408> (1035556259417571408)