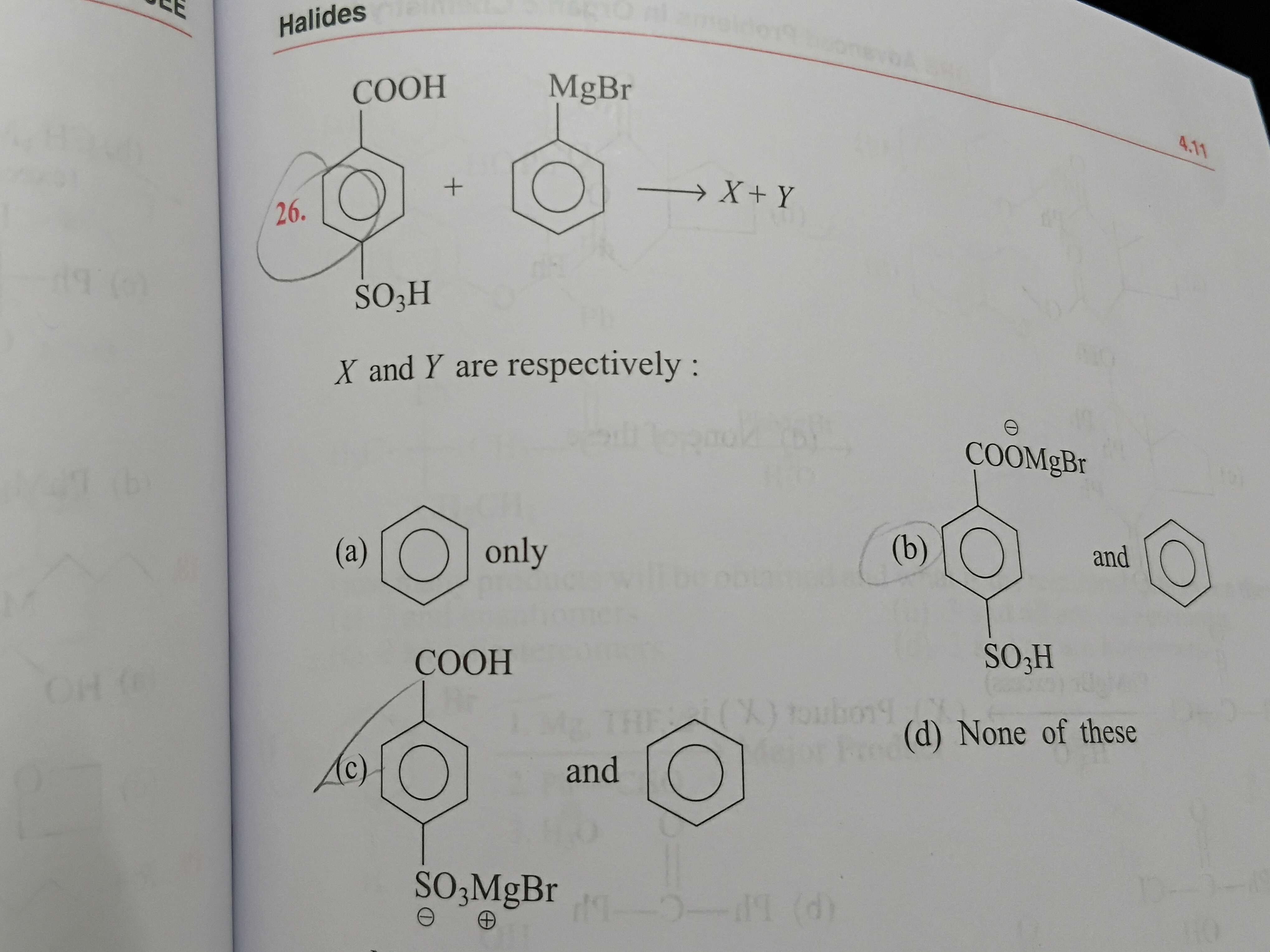
cooh so3h
Why b and not c? And what's the difference since resonance structures will have the negative charge delocalized on all the same atoms.
24 Replies
@Dexter
Note for OP
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.grignard prefers carboxy more
the double bond on c is more susceptible than the one in so3h
but ph-so3h is more acidic than ph-cooh and also so3- provides for better resonance than coo- so isnt the so3h more acidic
the c and o bond can protonate it and it is more acidic than so3h
no
cooh is more
no thats not true
wait what
let me look up ka values

and its kinda obvious too since there are 3 Oxygen atoms in so3h wheres 2 in cooh so better resonance...which leads to the H being more acidic
is it specifaclly for aromatc?
no
we have to see aromatic i think
arre just draw the structure
you'll see that the negative charge is more delocalized
in R-SO3H
than in R-COOH
that i know
wait i will open the book page in zumdhal and see whts given
ok
ok wow it had nothing let me see clayden
there is nothing particular i could fnd that would help
ill think on this
k
still open to answers
(please)
@Augustine it should be C
You are thinking in the right direction
And I don't find any flaws in your argument
Considering how strong of an acid sulphonic acid is ...
I don't think any other carboxylic acid could come even close to it while reacting with a strong base like grignard
I’m now sure it is C
I’ve asked some people as well
ohkk thanks a lott
himanshu pandey seems to have lots of errors 😭
Uhh koi ni
Yahan daal diya karo
yepp :)
Mark it solved now I guess
If you're done*
+solved @Varun_Arora
Post locked and archived successfully!
Archived by
<@1199636217302618184> (1199636217302618184)
Time
<t:1749245202:R>
Solved by
<@984016629119713290> (984016629119713290)