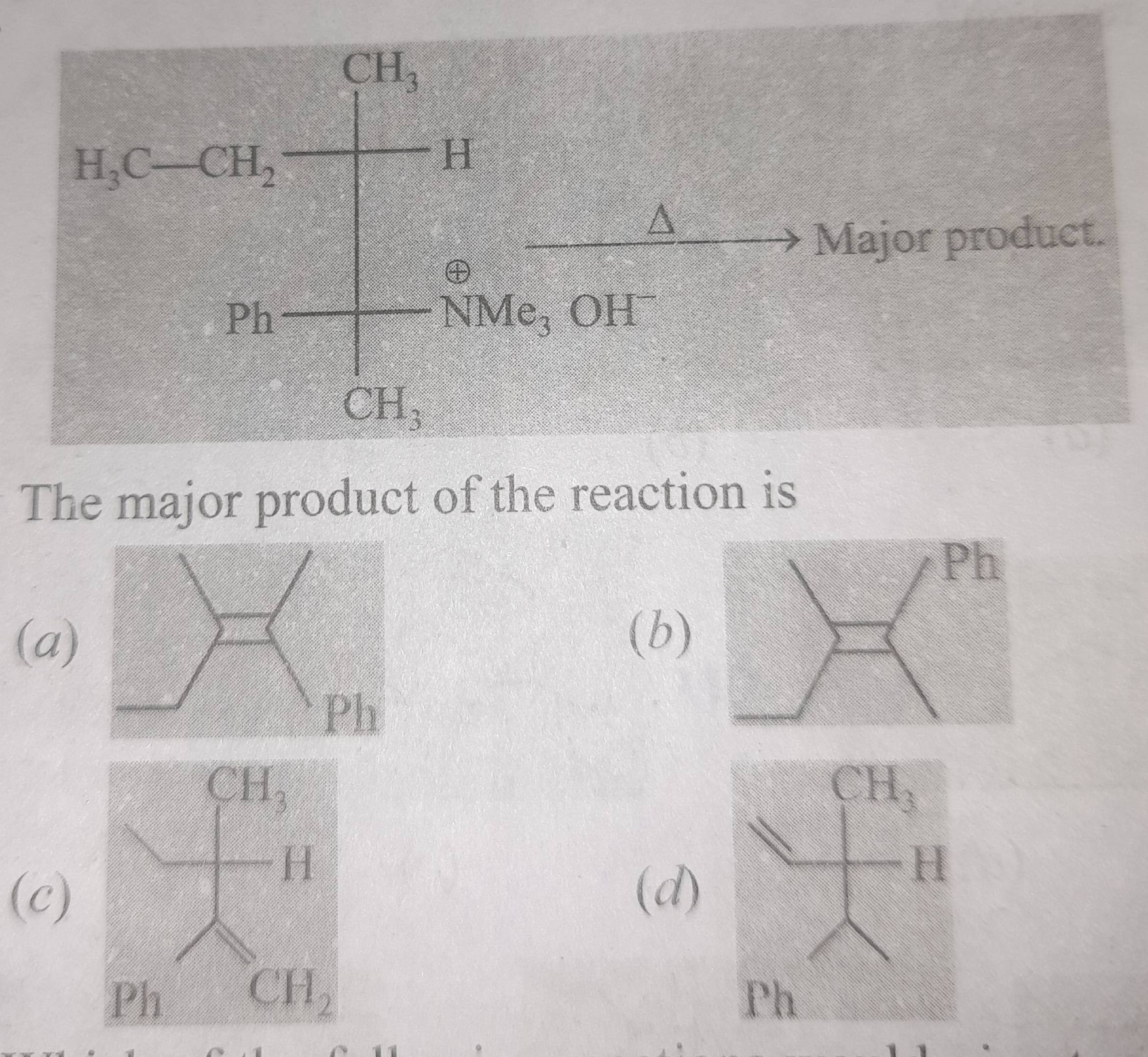
55 Replies
Aye Hoffman elimination
@Dexter
Note for OP
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.Beta H nikal jisme less stable carbanion ban rha hoga
Ye pata kaise chla bhai tereko
💔E1cb mech vro
Hoffman elimination hai
Yar main na reagent dekhke check krta konsi rxn
Isme to pta hi nahi chlra
OH- Nme3+ hai na
Heat karoge to OH- H leke paani nikal lega
Isme less acidic H lega
Hoffman elimination
Aur NMe?
Wahi eliminate hoga
Leaving group
Acha oh
Try kar
Haa dekhta vrna krta ping
Yar mera to A option aara
Ans C ha
@Dexter
Ruko
Dekh Nitrogen se gin na start kar.
Beta elimination hai.
Two beta H hai
Ek upar wala H.
And ek neeche ch3 se ek H
Zyada stable carbanion upar wala hona chahiye to wo nai niklega
Neeche wala carbanion zyada unstable hai to usse remove Krna hoga
Usse C aaega
Bhai pura smjh aagya
I was about to type but looks like aa gaya
Man identifying these rxns really is hard work
Just notice acids and bases
And electrophiles and nucleophiles
And logic se jaayo
Rather than trying to "remember" reactions
Yar electrophile and nucleophile to har rxn mei hi hote
Acha isme logic se mereko ye smjh aara, NMe OH ko nikalna ha to OH vala part pehle niklega, aur ab ye khud ko stable krne ke liye paani banana chahega, ab carboanion ki stability dekhlenge ki konsa H jayega
Exactly vahi identify karne aane chahiye
Aise hi logically sochna hai
sorted?
Yea yea
+solved @Dexter
Post locked and archived successfully!
Archived by
<@1382187168230936577> (1382187168230936577)
Time
<t:1750125225:R>
Solved by
<@1095655769376034818> (1095655769376034818)
@Prasan
@hardoc
@hardcoreisdead
idhar aaja
generalize nahi karna
Yes
to fir tune kese bola hoffmann ha
Look at steric crowding
And other factors
It happens to be hoffman
Agar wahan peeche ethyl ki phenyl hota toh wahan major ban jata
dekh jo H niklega
vo 2 jagha se niklega
ab upar vala H jispe ethyl ha, vo +I laga raha ha, mtlb jo carboanion banega usko aur unstable
to ye hoffman nahi ban na chahiye?
Yes , making that H less acidic
yar ekbar shuru se smjha do
mix hogya sb kuch
@hardcoreisdead
@Enamine
Bas aaya saar thodi der me
Bas chand minute
koina koina karlo
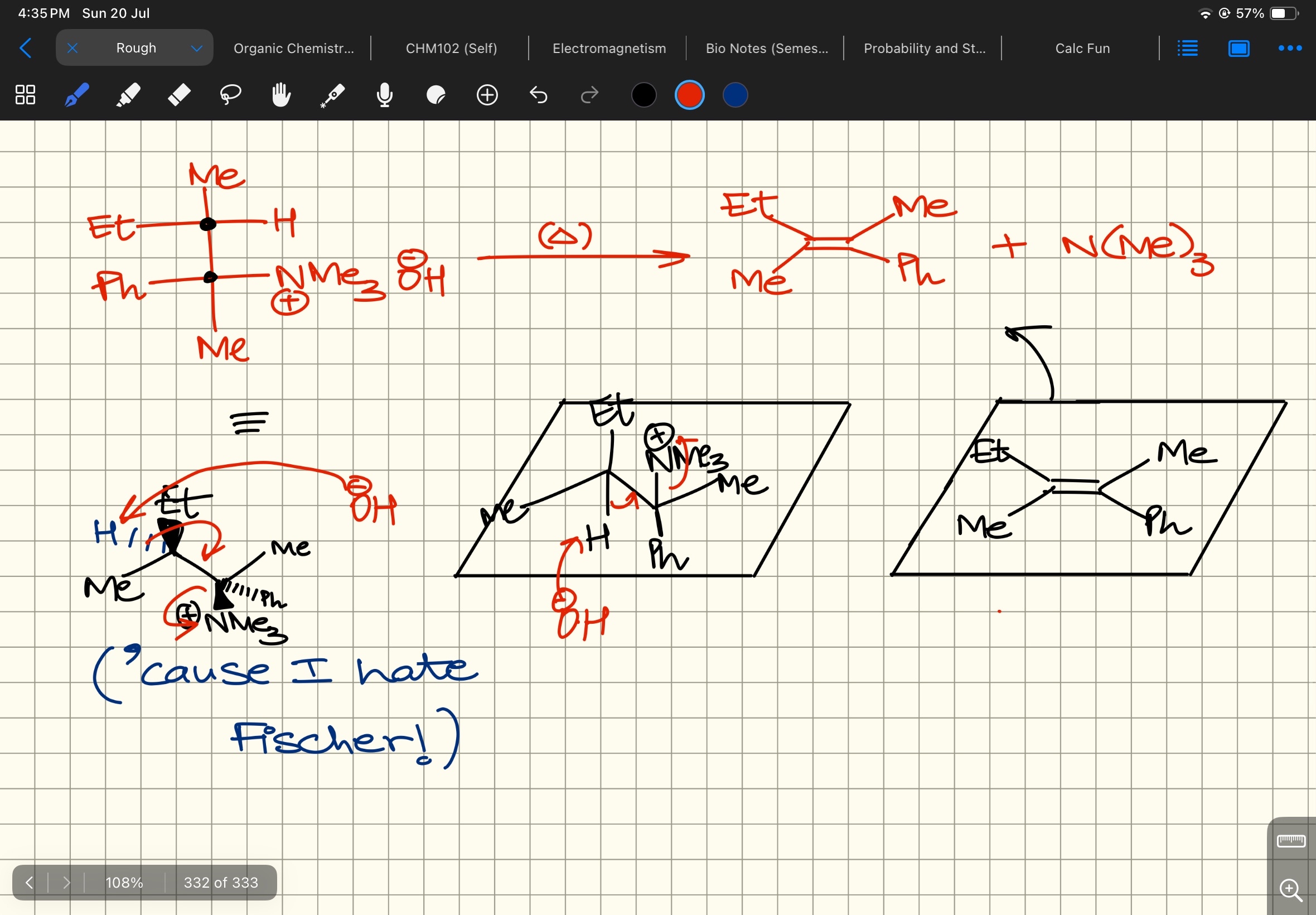
Yahan carbanion nahi banega
Concerted process
Vaise ek second
Even if it does from then it’s alright
Okay
N(Me)3 withdraws electron density really good
H must be closer to it
@Dexter tumhara C kaise aaya?
Hoffman elimination ka result h ki less stable alkene banta u
Hai
How do you know hoffmann ho raha hai yahan
There must be a reason
mhm i forgot the reason but ye wali rxn hoffman hi hoti thi iirc
Dekho acidity of those 2 hydrogens
Not very different
Dono me -I
Except 1 is primary
The other is secondary
Primary vala is more acidic
If that is the reason, then well and good
Otherwise there is some issue with this
accha
well💔
Shukar ha mera doubt pucha kisine
Maine kyun poocha tha @Prachi ji
‘cause driving force is NMe3+ ka leave karna
Usse pehle decide karegi acidity
Usme jyada difference hai nahi
So more stable product ki taraf
Acidity mei zyada difference nahi hai matlab ?
Ki primary aur secondary h se acidity difference nahi pata chalta ?
Chalta hai yes but inductive isn’t as strong
Resonance hai nahi yahan
Dono me hi
The one I drew is a more stable one
I’ll let you guys know
I’ve posted this somewhere else too
@Prachi @Prasan @Dexter
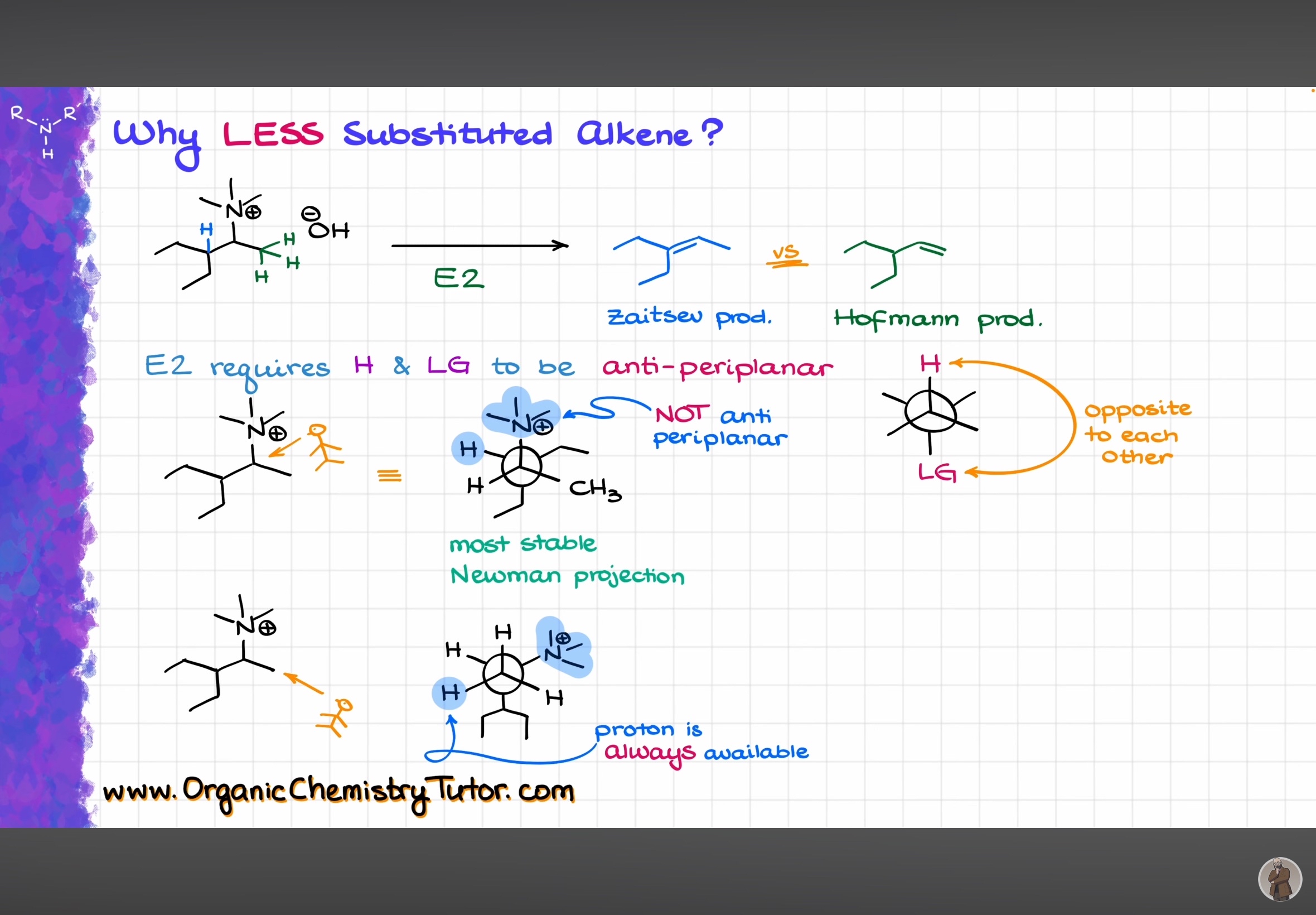
Credits : Victor Organic Chemistry Tutor
@Prasan can we mark this solved now?
+solved @Enamine
Post locked and archived successfully!
Archived by
<@1382187168230936577> (1382187168230936577)
Time
<t:1753268723:R>
Solved by
<@984016629119713290> (984016629119713290)
?
ye lock kyu ni hua
@Moderator
+solved @Enamine
Post locked and archived successfully!
Archived by
<@877351272251023440> (877351272251023440)
Time
<t:1753293744:R>
Solved by
<@984016629119713290> (984016629119713290)
Mehhh
Tf
abhi bhi ni hua
:uhh:
Bruh
+solved @Enamine
Kal interview hai uske baad weekend pe dekhta