39 Replies
@Dexter
Note for OP
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.Br2 is selective
Attaches Br radical on the most stable position
Basically Jahan sabse jyada stable radical bane
x>3>2>1
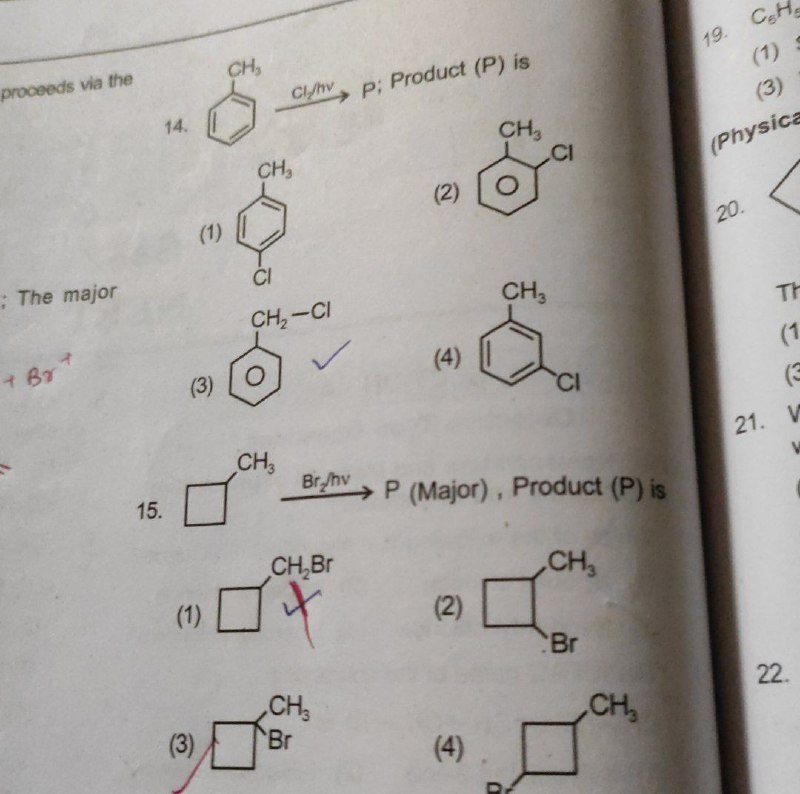
order ?
3 2 1 yes
Sabse stable tertiary
lekin iska radical kyu banega? hv se to halogen ka banta haina?
well agar benzylic ho to?
Mechanism yaad karne ki koshish karo…..
Yes tab vo
to 14 ka ans c kaise?
Dhyaan se dekho
Benzylic konsa hota hai?
Benzene se directly attached
Benzene vaale to saare sp2 hain
sp2 is electronegative
Carbocations and radicals are not stable there
only carbanions stable?
sp2 and sp ka s character jyada hai
Bent’s rule yaad karo
More electronegative
More s character
ye kya bala hai ab'
:uhh:
…….
😭
11th class
Chemical bonding
nhi yaad
revision karna hai
Dekho for now remember
S character
Jyada hai agar
Tab electronegativity jyada
sp carbon > sp2 carbon > sp3 carbon
Electronegativity ka order
Thik ?
okkkeyy
likh leti hu
Hmm tab is logic se socho
Sp2 pe to stable nahi hoga
Bhaari electronegative hai
double bond kyu hi dega
Yeah
To BAs benzylic pe ho sakta hai
okey okeyyy
Or niche vaale me tertiary
matlab aise free radicals me most stable wala banega
Yeah
okey okeyy
got it
Ye GOC me nahi padhaya kya?
S character vagahra
yaad nhi hai agar kiya bhi tha to
Like you studied from lectures right?
11th me padha hi bhul gyi hu
revision nhi kia hai 11th organic ka
Hmm okay koi ni
Dheere dheere you’ll get it
ye bhi karenge ab
GOC and chemical bonding padho acche se
Needed hai
ok list me likh leti
Pehle chemical bonding must
Uske baad GOC
vo adha yaad hai
adha nhi hai
Reaction part saath saath chalta rahega
Koi ni ho jayega
okeyy okeyy
Alright chalo mark solved now I guess
+solved @Enamine
Post locked and archived successfully!
Archived by
<@888280831863451688> (888280831863451688)
Time
<t:1750328451:R>
Solved by
<@984016629119713290> (984016629119713290)