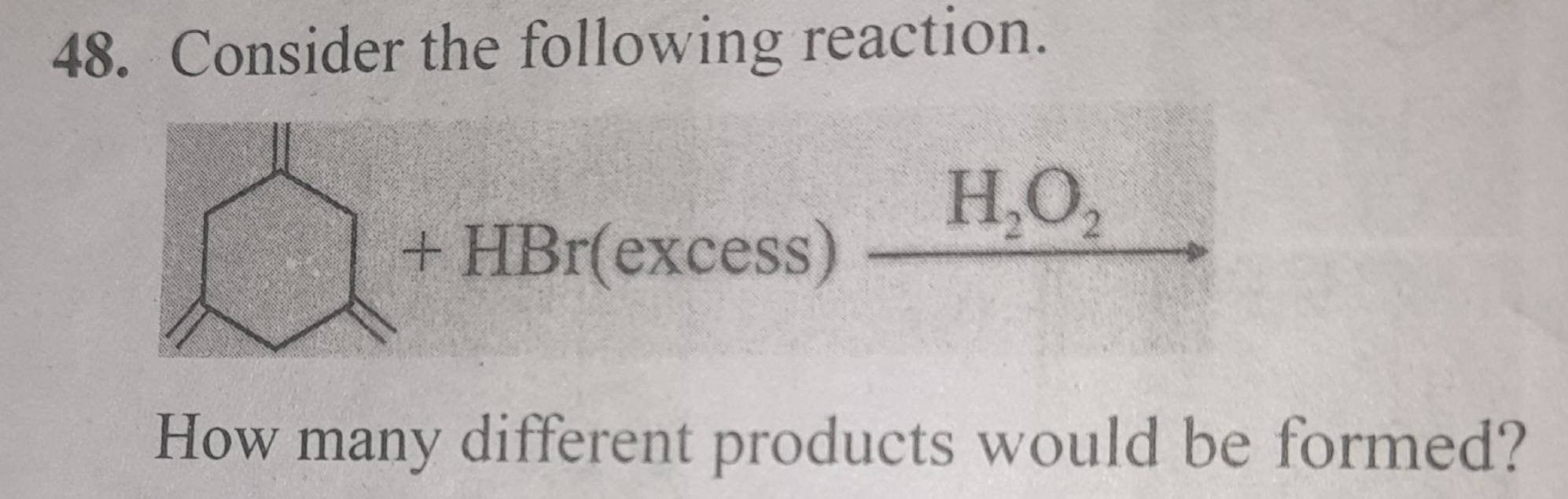82 Replies
@Dexter
Note for OP
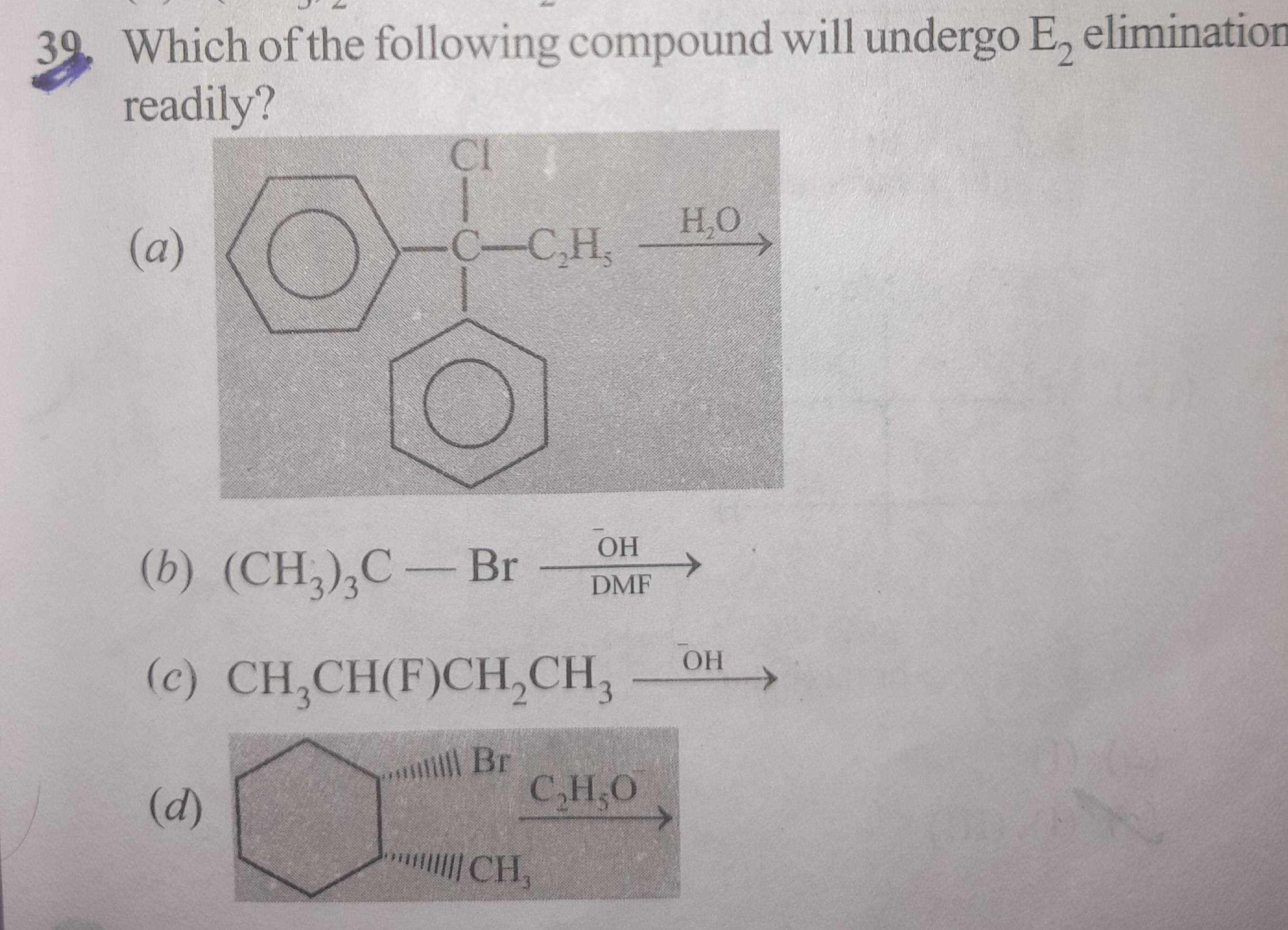
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.first one d?
goal is to find the ones that would undergo e1 ie carbocation formation
for first one
i thought that we should look for a strong base
bcz strong base generally do E2 elimination
same idea for 3rd one. Sn1 is what happens
carbocation stability
again d?
yea you can go down that route too, but b and c also have strong bases
you have multiple factors na
strong base is one of them
but in b we have PAPS which goes sn2
yea cos there is no H to remove :)
ouh
mb bruh
so we should look for sn1 you say
please ek baar sabke options ka reason bata do
E1 not Sn!
same reason for all
carbocation is stable in a
and c
b no elim, stable carbocation, sn1
second question let someone else do, I do not have fun counting things :P
whts ur work on second one? can u share
plus it is peroxide in there so anti markonikov rule
and it is excess also
c vala carbocation and d vala dono secondary hain, so how did you differentiate b/w them?
Stereochemistry :)
Do you remember the mechanism for e1 and e2?
www.google.com
Mechanism of the E2 Reaction – Master Organic Chemistry
Found on Google from masterorganicchemistry.com
Needs to be anti for e2
Teeno jagh lag jayega Br
Cases consider krke total count krna pdega
Abhi bhi teeno me dikkat hai ye kuch ho gaye @Prasan ?
teeno mein hi ha
Dekho 39 me you should look for a strong base
E2 hai
Secondary carbon hai
yeah so c and d mei problem aarahi usme
Strong base hai
E2 would dominate
C me the base isn't particularly strong
ouh i get the reason now
RO- stronger hai OH- se
c2h50- mein c2h5 +I lagaraha ha
to elec density badh rahi to nucleophile aur badhia hota ja raha hain right?
I mean.... haan thik hain vaise ye reasoning galat ho chuki hai aaj kal 😭
koina apne liye to yahi ha
You can say O- pulls electrons from Carbon
to kyu bata rhe
🙂
yebhi theek h
Ye bata raha hun ki vo galat hai to mat use karo 😭
oh ohhhhhh
😭
Alkyls are not electron donating
Via inductive effect
Hyperconjugation se karte hain yes
Kisi more electronegative group ke saath lage hon to karte hain yes
okay
acha 39 hogya
next
Now 48
Isme teeno pe ethyl right
Or teeno chiral hain
Nahi hain ruko ek sec
So dekho
HBr/H2O2
Usse bahar side OH lagega right
?
yes
To isme basically ye banega
Ruko bana ke bhejta hun
ruko yaar aap online hi ho to mai bhi vo 3 post kar deti hu
Mujhe jaana hai dost ke birthday pe 😭
varun bhai saving my chemistry everyday
Tum bacha rahe ho bhai khud padh ke
Mere hisab se ek hi ban Raha hai yaar
Product
ans shayad 3 ha
Let me see again
Ruko
kya karoge moh maaya me reh ke padhai karo aur kya
😭
jkk
Apni padhai to kar bhi nahi raha mai 😭
Agle sem ka padhna nahi chaalu kiya abhi tak 🤓
Bol rahe hain mushkil hai
Or maine to combination of subjects bhi itna unorthodox liya hai
to karo phir
Chemistry maths 🤓
ewwwww
math is so eshh
Maths is lob
Don't say anything for maths
doubt pe focus krte ha
Bhaiii
What was I even saying
Tumne correct kyun nahi kiya mujhe 😭
Andar vaali side Br lagega
HBr se reaction hai literally
andar vali side?
Mera net kharab hai ek second ruko bas
Aa rahi hai photo
okay
Ye dekho
Aa gayi kya?
kitni aur der
Photo?
nahi aai nai
Yaar mai kya karoon 😭
Net kharab hai to
naya network leo
Ji bua ji jarur
kewl
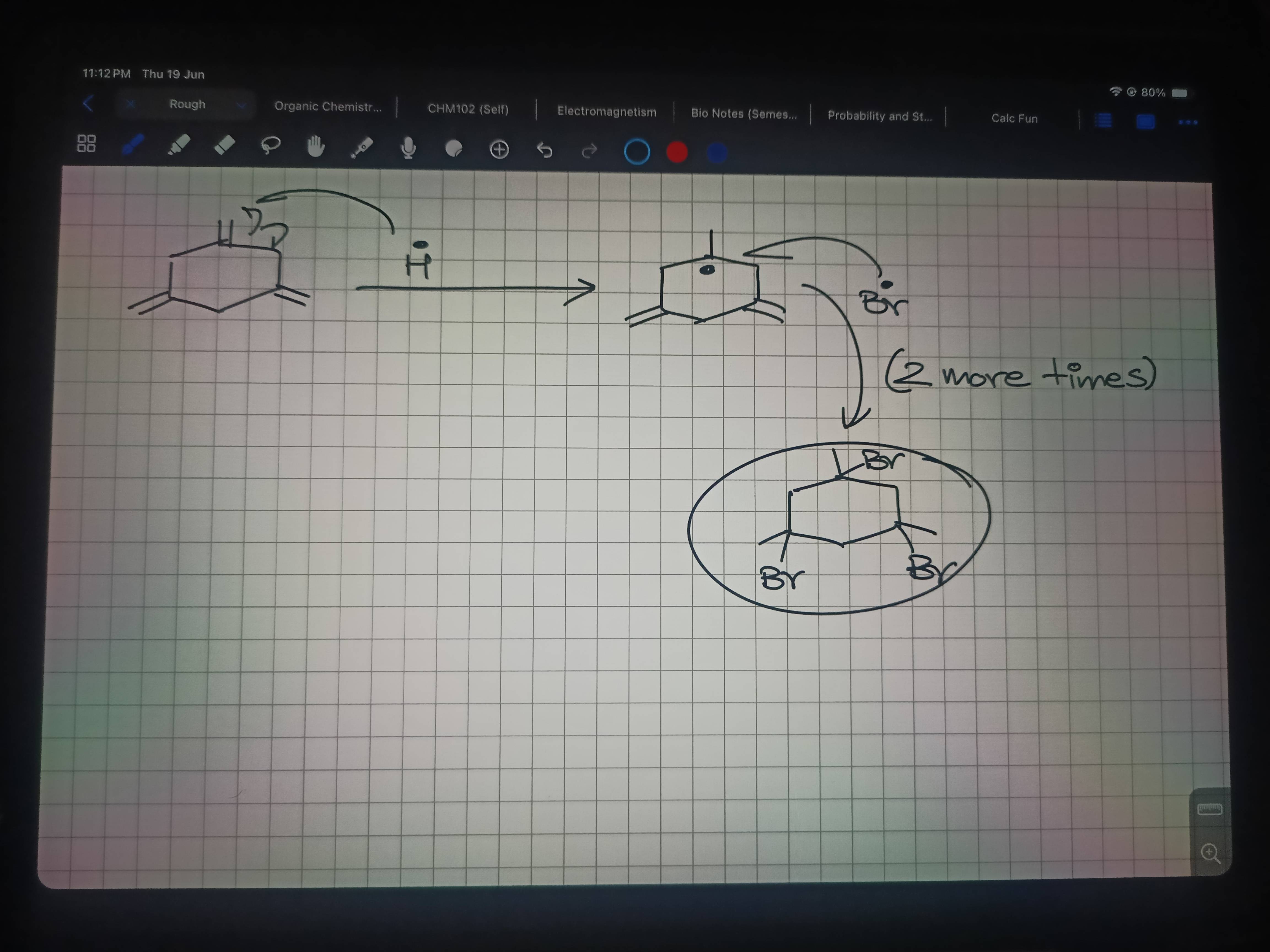
Does this work
Dekho ek baar
Ab isme notice karo
OHH
kharash effect ha kya
Haan
are shit yar ye to dhyan mei nahi aya
chlo 48 bhi hogya
last
to maine markonikov bola to kyu nhi dekha 😭
Usko free radical dekhne the
yes
welp
jaari mai 😭
Ye dekh lo 3no products
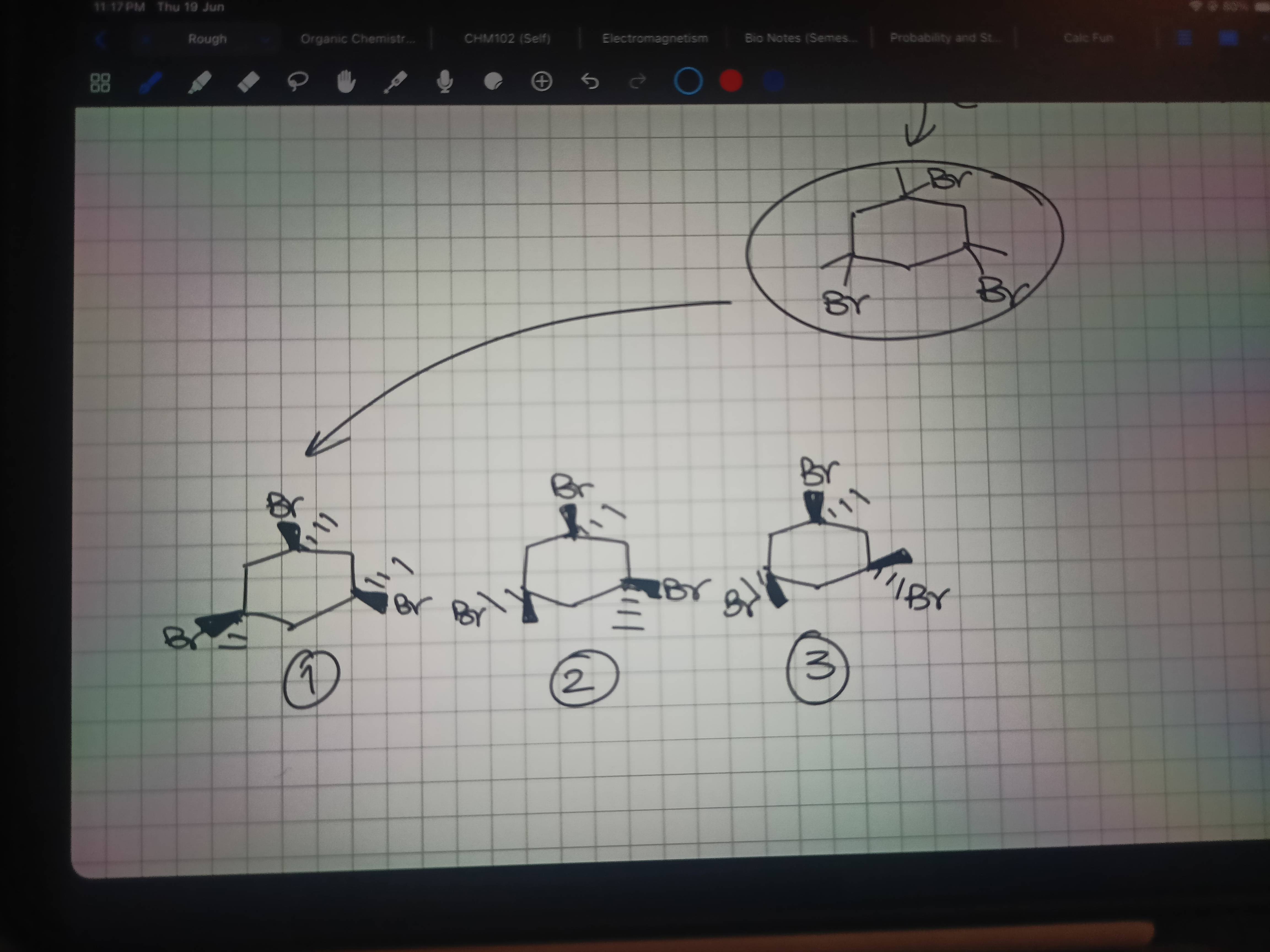
Agla dekhta hun abhi
Jaayo jaayo 🙂
Tumhare doubts dekh lunga sone se pehle dw
theek ha
Dekho
Last vaale me
Alcohol hai
SN1 karega
'cause it's a weak nucleophile
acha
To carbocation ki stability dekho kisme max hai
got it
thanks bhai
Not a problem
Btw sir ka point bhi dekh lena I just saw
Base is not the sole criteria
Primary Carbon hota to SN2 karta d option
In the first question you gave
okay
close krra hu
Hmm kardo kardo
Uska article I'll see now
+solved @Enamine @iTeachChem
Post locked and archived successfully!
Archived by
<@1382187168230936577> (1382187168230936577)
Time
<t:1750355497:R>
Solved by
<@984016629119713290> (984016629119713290), <@1035556259417571408> (1035556259417571408)