14 Replies
@Dexter
Note for OP
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.Multiple answer ha iske
@hardcoreisdead
B c
D not sure
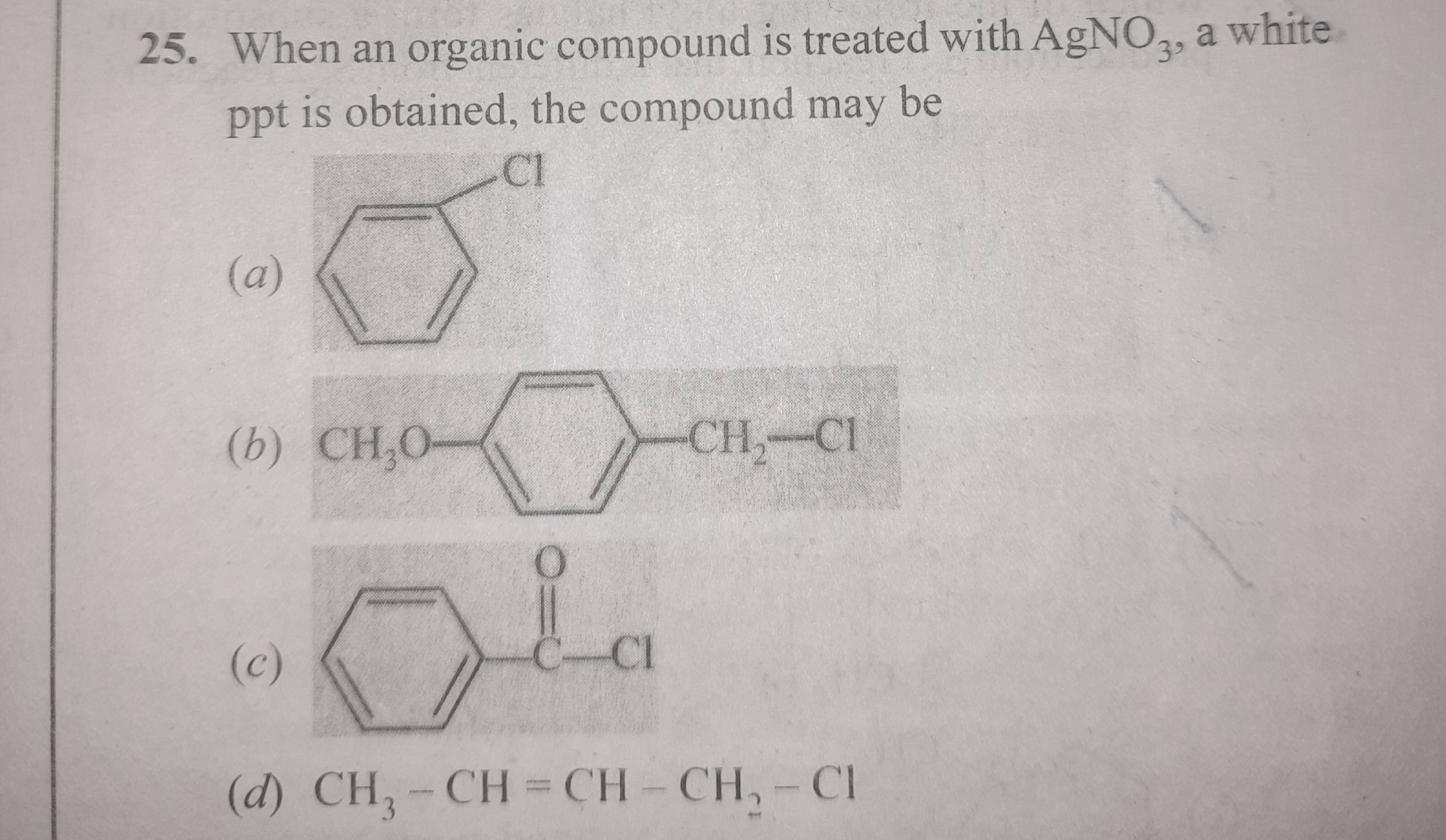
This has to be a double displacement rxn
So if an ion can be formed on the R group
That’s referable.
Preferable
B and c form r+ ions. A and d do not.
Bcd is the answer given
b and d then
eh yeah C can ig
oh shoot my bad didnt see the double bond on the carbon. yes b, c, d
allyl carbocation would be stable.
So we want a stable carbocation for ppt
C has the ability to back donate that lone pair from oxygen to carbon
That directly provides a lot of stability
A me it basically can not form
'cause it's a "phenyl" carbocation we are talking about then
Positive charge on an sp2 carbon
Vaise to even D is highly unlikely but still could be formed to some extent
ohh right
^
sb smjh aagya
+solved @iTeachChem
Post locked and archived successfully!
Archived by
<@1382187168230936577> (1382187168230936577)
Time
<t:1750444978:R>
Solved by
<@1035556259417571408> (1035556259417571408)