120 Replies
@Dexter
Note for OP
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.The only thing I don't understand is why is the positive charge not on the 3 degree carbon
The attack could've happened from the other carbon as well
Omg
@iTeachChem sir is that carbocation position cuz of dancing resonance
(sorry for pinging)
Man it might be tho
Would make it a good question
No clue. Organic I am not the best person
Ah kayy
I want to get to the bottom of this
@Opt what do you think
This is just NGP isn't it?
Yeah but it could've attacked from the other carbon too right
On the benzene
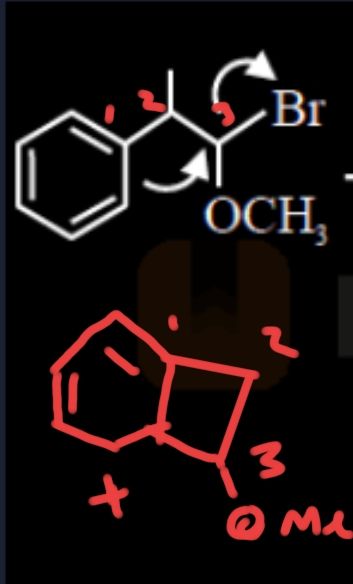
Like this i mean ( I missed a methyl in position 2 mb)
That specific posn looks similar to that in cyclopropylium
Ofc the product is same
I'm talking about the intermediate
Well I suppose that it's just figurative. The plus charge will be delocalised along the entire benzene ring
It's not that the charge is on one specific carbon
True
Which intermediate is favoured then
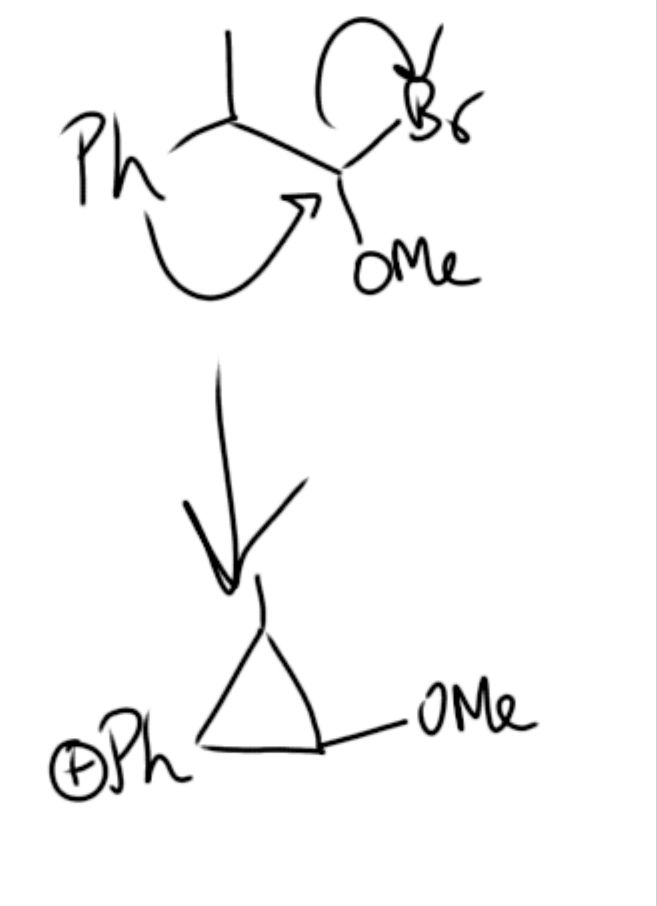
Abuse of notation but whatever
ye kya hora hai
mujhe to kuch smj nhi aaya
and why is there a ring formation
3 membered to unstable hai kya?
@Enamine (sry fr ping)
Heyy
Ek to ye ping karke sorry mat bola Karo kuch nahi hota 😭
Ye same question somebody asked before
Prasan ne hi poocha tha
A pathway more favorable than NGP (the kind of pathway shown here) would be.......
(I'm sending a pic wait)
Kind kind
Why can't send a photo whattt?
That plus vala sign isn't working
f
Must've crashed
I'll restart waut
Wait*
Working fr me
Yes
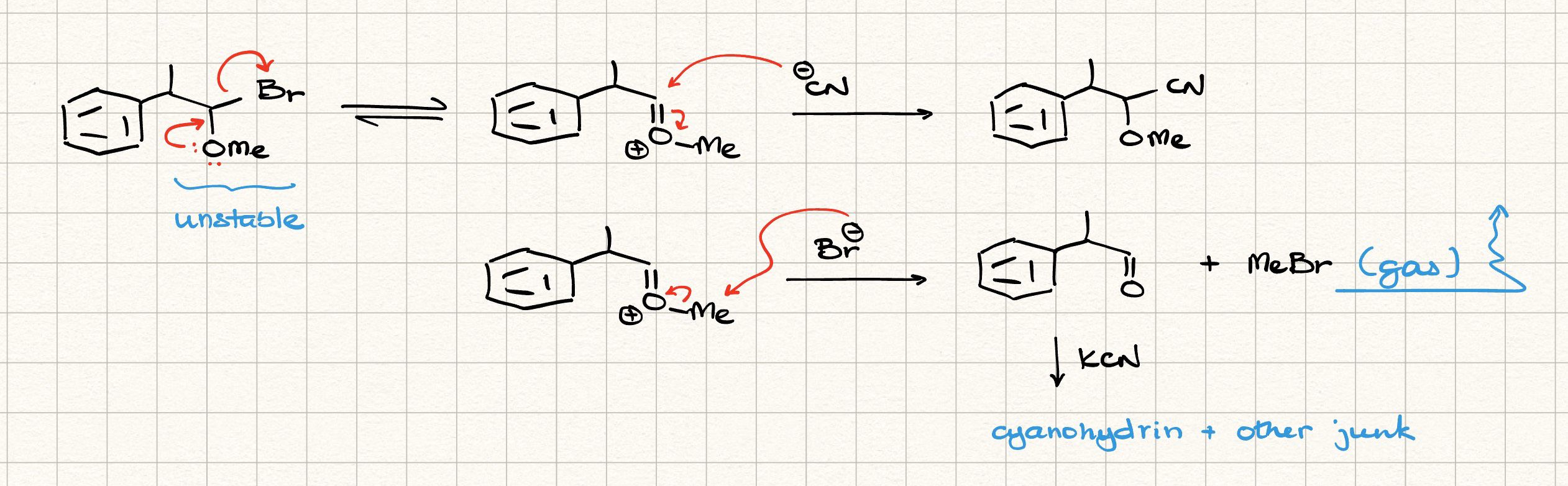
Worked
The given mechanism is basically wrong
This one isn't
Jo Maine bheji
Why didn't I think of that before
Haan matlab in the question
'cause that doesn't generally happen very easily
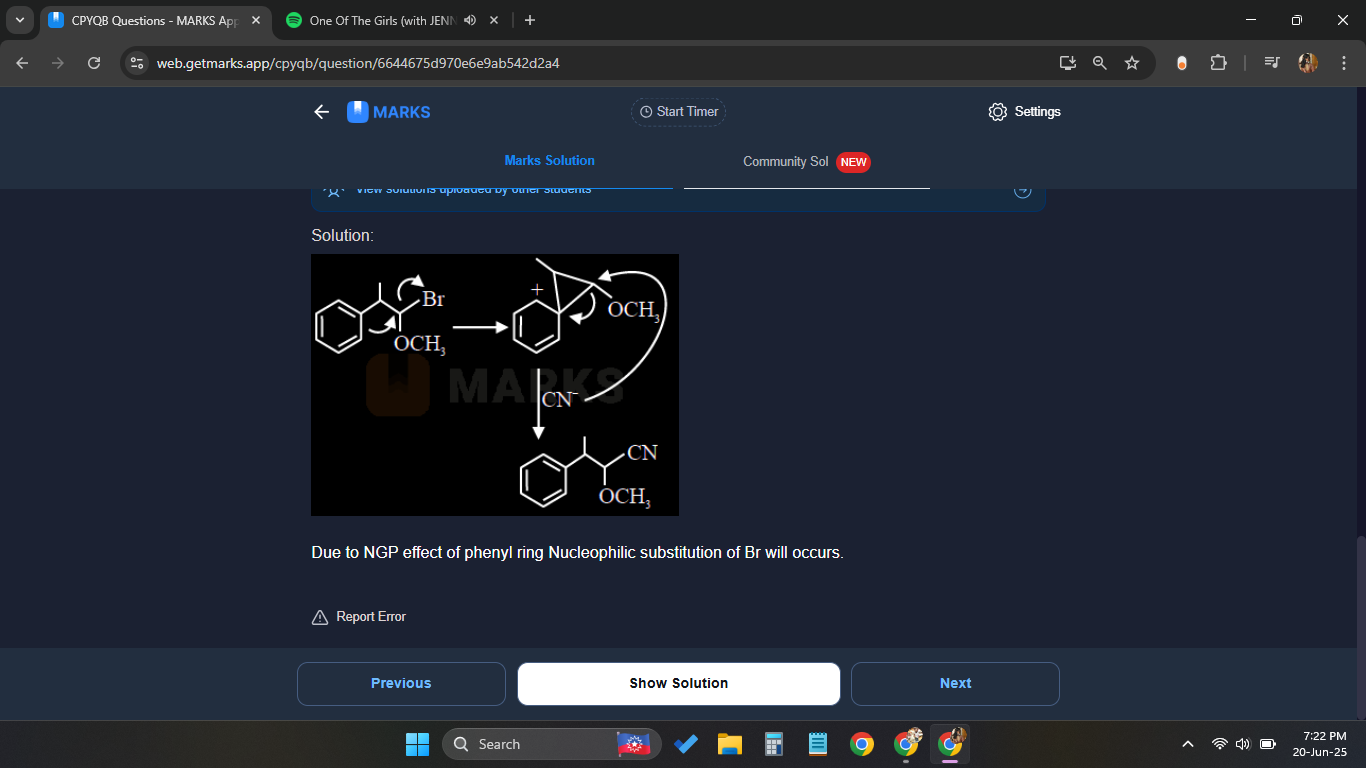
Answer provided by matks
Marks
You know I did the same thing
I got stuck basically
At the step in which Me is taken up by Br
Right tho I think I have seen this before
wait what toh isme ngp nhi ho rha?
Electrophilic substitution types hi hai
Thoda sa
Ahh
Hua na
Why would you form an anti aromatic moeity agar usse better pathway se jaa sakta hai to
I got excited man when I saw possibility of dancing resonance
Ek to ye mat bola Karo use please 😭🙏
It's a special type of p hyperconjugation
Resonance dance nahi karti 😭🙏
ngp is like 1000-2000 times faster and uh its been observed in similar reactions
Mb
Sahi info toh mil gayi
It is yeah but here the "reasonably" better pathway without a strained anti aromatic intermediate is the one I sent
Sab victor ochem tutor ki den hai
All hail to him
Bro has handled organic chemistry for me
So well
Op stuff fr
That mechanism btw
Which I sent
I asked him on his discord
Unhone hi draw karke bheji
Oh damn
Ochem tutor himself
Yup:)
fan moment
Op
There are two of them
Which one do you think I'm talking about
🙂
The one who teaches on the black screen?
Nope
There's this channel called
"Victor Organic Chemistry Tutor"
Bro is goated fr
Damn
Gotta check out his work now
Sure do
He has a discord too where he usually does answer queries
He's fed up by mine now though so tends to ignore 😭
lmaooo 💀
:psyduck:
Bhai hamari convos have gone like hours long
About the same thing
ngl organic can be very interesting if u have a good teacher
Bro just says I'm done "indirectly" after a point
Honestly, that's the case with everything
true
lekin jo unhone explanation diya vo kaise work karega?
Bromine badhiya leaving group hai
Nikalne pe stable carbocation
Directly back bonding oxygen se
but 3 c ring isnt stable right? then why
3 carbon ring kahan banayi
Vo vaala galat hai
Marksapp vala
..
sar phod lena chiye apna
Yeah this one ain't right
Aree 😭
😭
ik to aise hi meri neck dead hai
Itna mat socho dw
aur back bhi
Answer isse bhi same aa raha hai
ab lagra sar bhi dead ho gya hai
a similar reaction
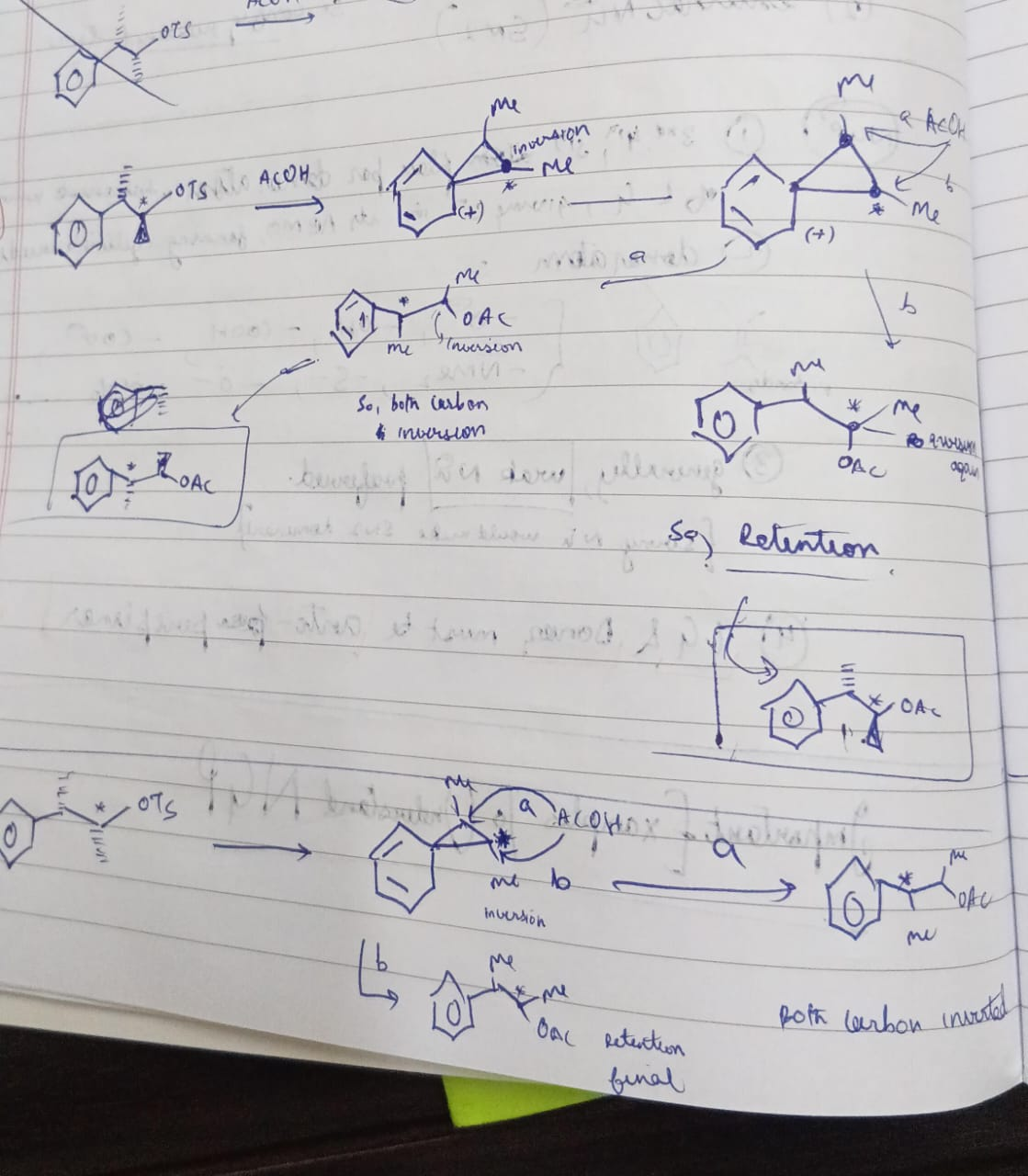
But the correct one is jo baad me bheji
again why 3 c ring?
WHYY
Here you had nothingbetter
ngp is faster and shit 😭
Actually ban ne me problem tab nahi hai kyunki cyclopropyl methyl cation ban Raha hai
Highly stable hota hai vo for some special type of hyperconjugation
(jisko coaching vaale dancing resonance bolte hain)
oml 😭
Yeah it is par energetically kya favourable hai vo bhi dekho na
Jab bina strain ke kaam ho raha hai to kyun banayein ring?
hn but voh toh practical data se hi pta hoga ig mostly
ExcTaLy
Strain yaar
Not favourable over getting a directly coordinated pi bond from oxygen
i mean acoh could have done sn2
What?!
mujhe kuch smj nhi aara
kya hora
What did you just say
Say that again and I commit suicide
💀 arre arre aisa kya bol diya
💀 🙏
AcOH does SN2?😭
acoh co-
😭
jkk
shit i thought etoh
mb
......
EtOH bhi nahi karega bhai 😭🙏
Weak nucleophile hai vo
why 😭
this is why i like standard names and not common name
Solvolysis prefer karta hai ......
strong base
hn but polar aprotic solvent ho
STRONG BASE
no heat
EtOH nahi hai strong base 😭🙏
.....
eto- hai?
ik hi baat hai mere liye
yh ig
like oh-
Nahi hoga tab bhi bhai
Solvent only "enhances"
Never "decides" the mechanism
i think abhi mera dimag placenta formation me jyda hai to organic me chl nhi rha
2 degree and 3 degree pe elimination karta hai to moderately strong bol sakti ho
to kya vo favourance me help karega?
hain? maine to suna hai ye kafi accha base hai
Agar strong nucleophile already hai or tab polar Aprotic solvent ho
To rate badh jayega basically
hm sn2 ki possiblility toh hogi na...albeit secondary carbon and weak nucleophile
Williamson synthesis yaad hai?
weird mech with nitrogen?
Nope
Nucleophile weak ho to kabhi SN2 se nahi jaana pasand karega
to form haloarene?
accha
Alkoxide reacting with primary alkyl halide to give ether
Williamson synthesis
maine alc nhi kiya hai....
Ohhh shit-
Sorry 😭
🙂
🔪
jk
JK
Mere dimag me saari organic same time chal rahi hoti
Mujhe chapterwise nahi yaad rehta
😭
shyd 4 mahine baad mai ye bol paay
tbh vaisa hi hona chiaye
Bol payogi bol payogi
Bas efforts daalo
hnnn ab aaj to bio ko diya tha
almost one chp done :coolfrog:
+solved @Enamine
Post locked and archived successfully!
Archived by
<@888280831863451688> (888280831863451688)
Time
<t:1750536000:R>
Solved by
<@984016629119713290> (984016629119713290)