25 Replies
,rotate
@Dexter
Note for OP
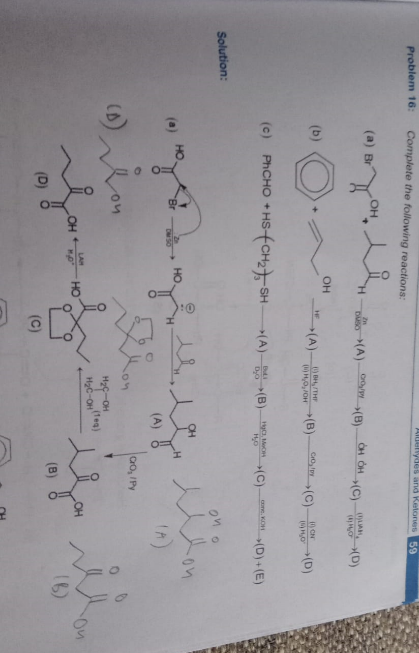
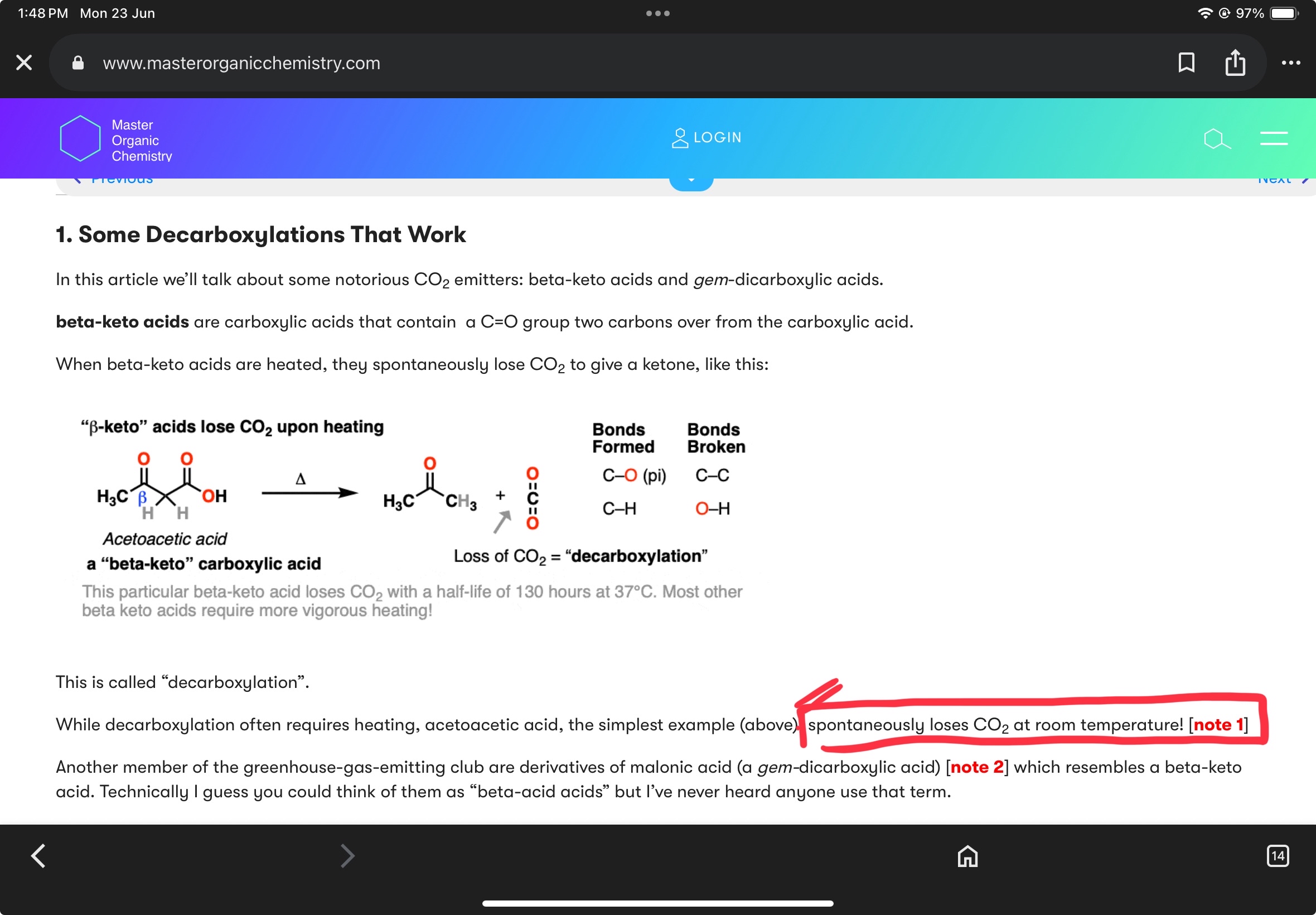
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.Shouldn’t that beta keto acid decarboxylate?
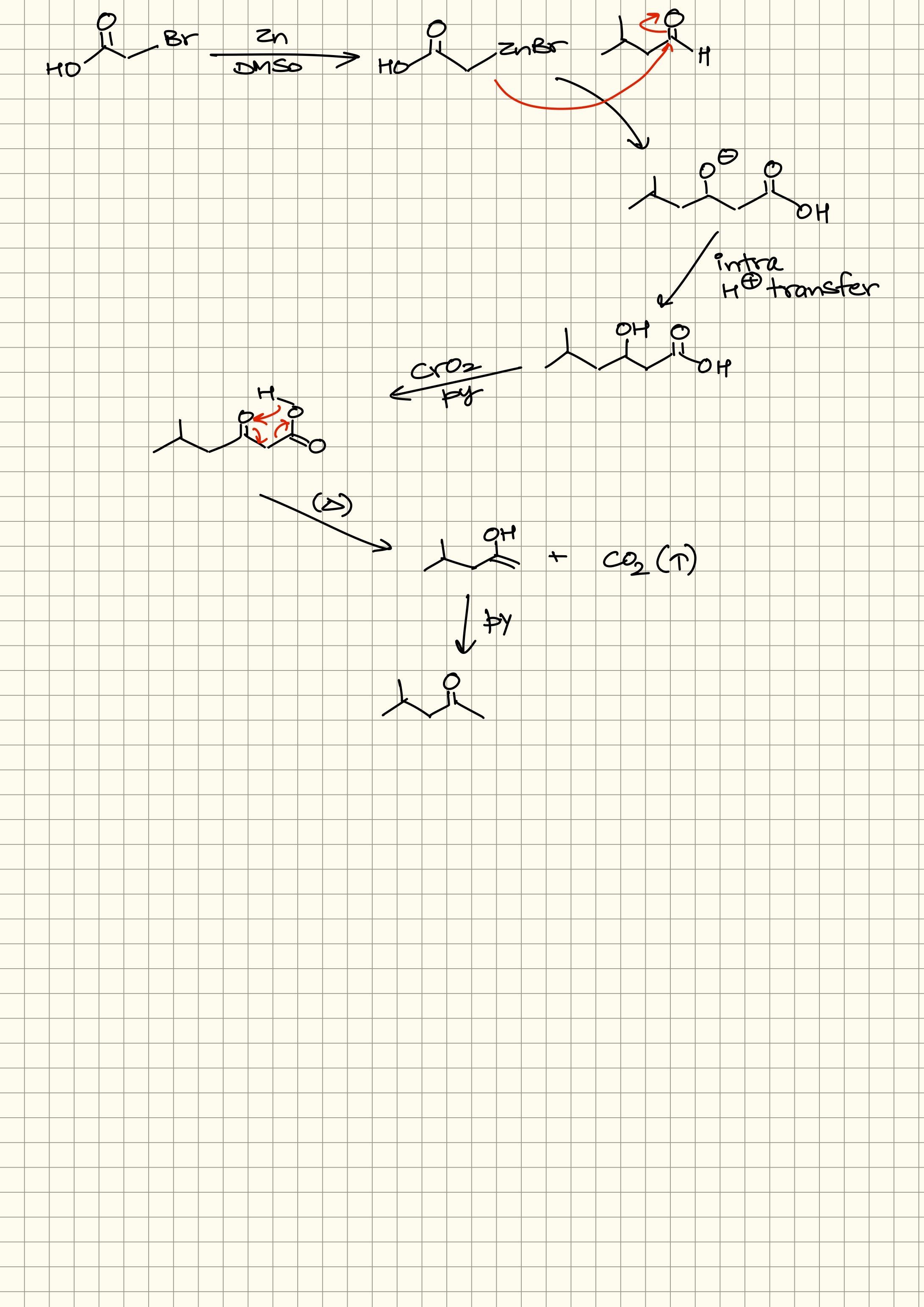
So yeah
You’d mostly not find a beta keto acid lying around like that
This is what should actually happen
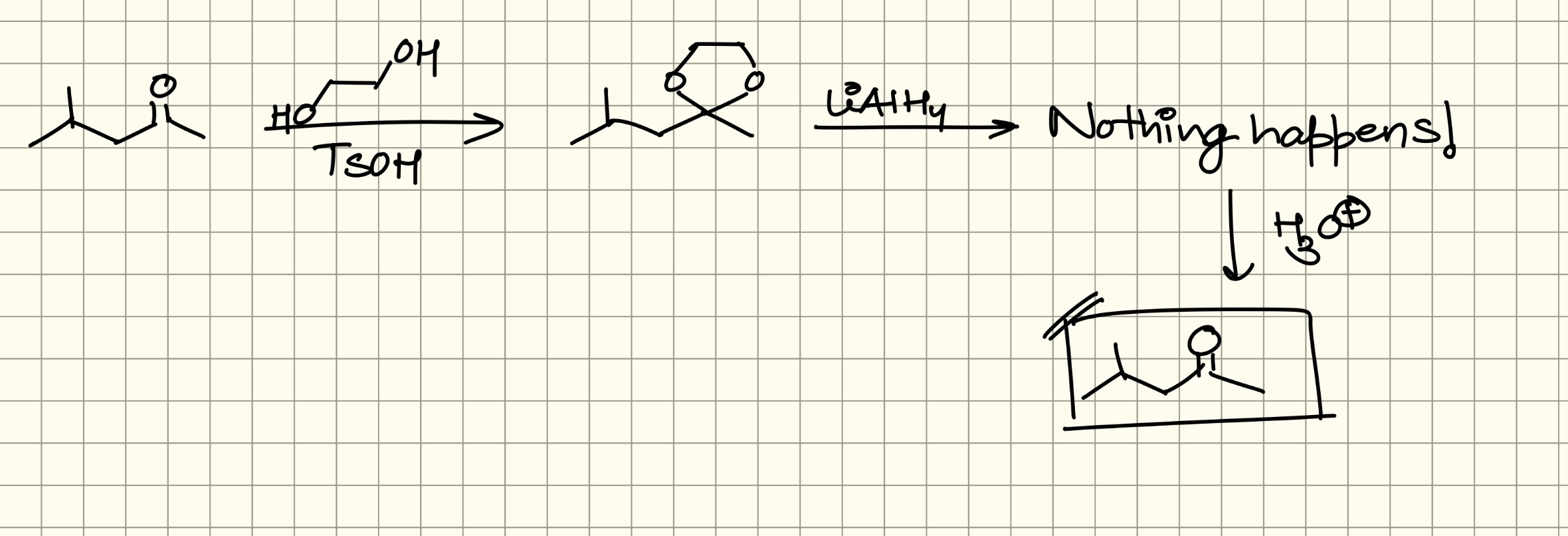
only upon heating
Ye dekho
The energetics of this reaction don't really need heating ngl
But haan
The question demands something different I guess
true
Actually me this works like shit
Kyunki socho
Agar decarboxylation nahi bhi hua
To there's glycol
Reacting with a freakin acid itself
Carboxylic acid hai
Won't that tend to giving you pinacol-pinacolone vala product
which step
Beta keto acid me glycol dala jab
Protection ke liye
han toh protection hi hogi
on the other c=o , not the cooh one
Are you sure?
COOH provides H+
Accha khasa vo bhi
-I bhi hai ketone ka
Accha khaasa acid hai
Protonation of glycol giving you a lot of pinacol-pinacolone junk
not what the ques want 😭
I mean meh
Ab options jo hain dekh le yaar fir usi hisab se
Ab mai kya hi bol sakta hun jab module banane vaale ne major possibility neglect kar di
so my prods right?
Yeah I guess
Jee ke hisab se
book ke galat hai?
Ek second
I just noticed one thing
Yeah okay okay
Tumhara vala thik hai
Last step me the book did some bullshit
Acid reduce karna tha
The book didn’t
+solved @Enamine
Post locked and archived successfully!
Archived by
<@741159941934415883> (741159941934415883)
Time
<t:1750745515:R>
Solved by
<@984016629119713290> (984016629119713290)