43 Replies
@Dexter
Note for OP
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.is a double bond more electron dense than c=o
what makes you say this? O is more electro-ve na. this isthe main reason behind all ald and ketone rxns at the C=O bond
so the electron density will be on the O, and will be higher there
i had the same reasoning
mcpba is supposed to attack on more electron dense site
so D banta
but C is correct
anyone..?
m-cpba did epoxidation of double bond if I'm not mistaken. (Idk the reason, this was just written in my notes ;_;)
yeah but why not the c=o
@Enamine apne dekha ye?
Actually nahi
Dekhta hun bas 😭
bas dekhna nhi hai
karna hai
Haan yaar karunga
Saare ek saath hi dekhta hun 😭
Bohot hain
ok okkk
Heyy @hardcoreisdead
I feel like it’s more about the product being formed
In the case where you got both ketone and C=C
The C=C forms a product with a relatively higher energy
strained
But the Baeyer villiger oxidation leads to a six membered ring which is to say the least strain free
So I think the answer should be ABD
https://chemistry.stackexchange.com/questions/134495/m-cpba-selectivity-between-baeyer-villiger-oxidation-and-epoxidation
I found something
Ngl though I’ve never encountered such a thing
The answer seems to say that it depends
if it were jee adv u wouldve gotten -2 for this
Bruh why?
u r the one whos supposed to ans
I didn’t check A and B ngl
I thought they were clear enough
Baeyer villiger hogi
B is wrong lol
Then it’s about selectivity I’m each
Hoga I didn’t check as I said
Mujhe laga vo to apparent hoga like
Structure me kuch galti ki ho to alag baat hai
Reaction was clear ki ko si hogi
That’s why just checked C and D
A is def correct since more electon dense site pe rxn hoti
B is another story
B me kya issue hai check karta hun
C and D mein bhi electron density ka relation hai
Ohhhh
Bridgehead
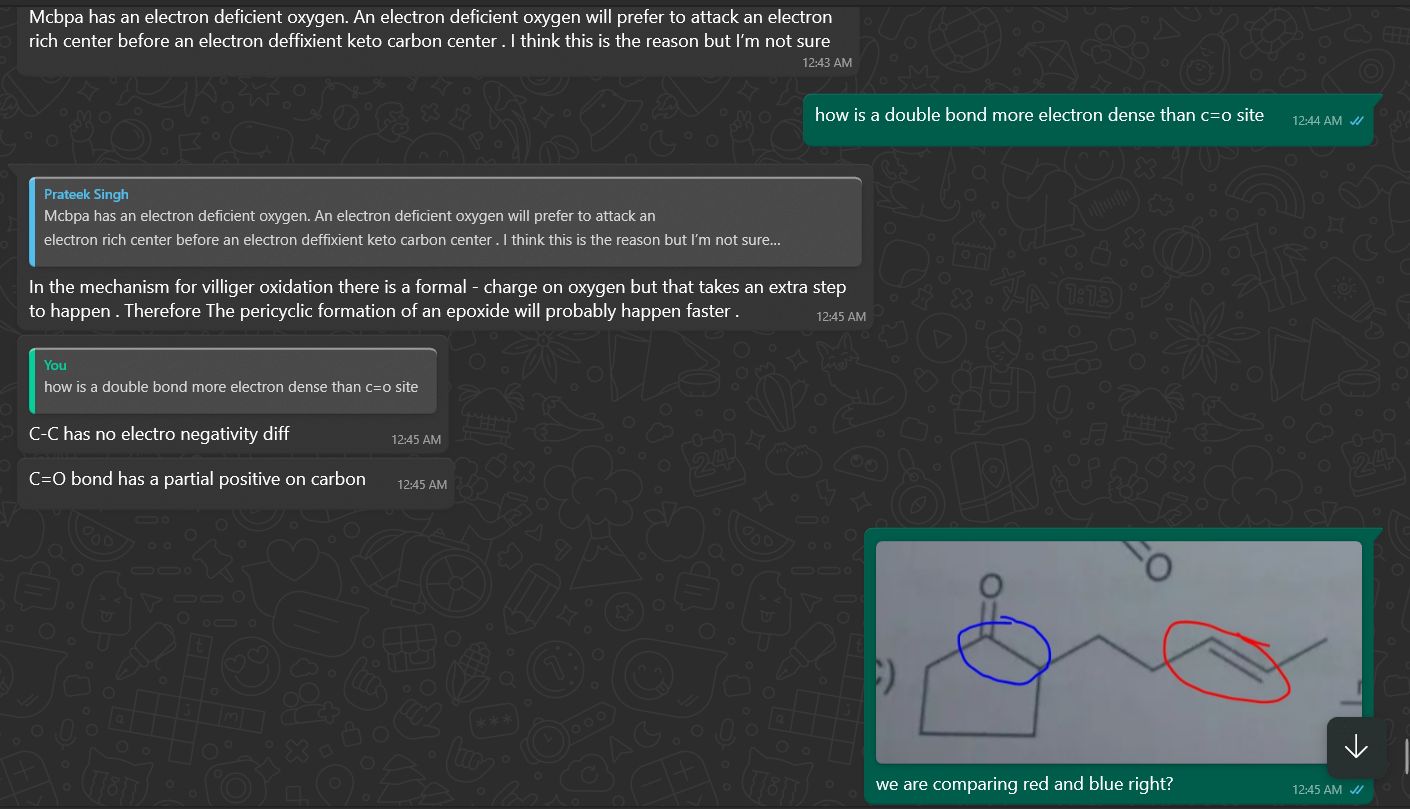
can we say red is more electron dense than green due to electrong withdrawing nature of c=o
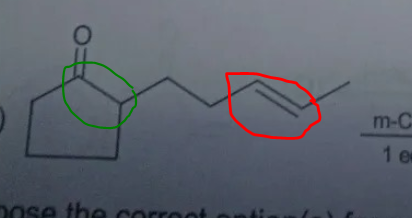
I guess aise hona chahiye B? @hardcoreisdead
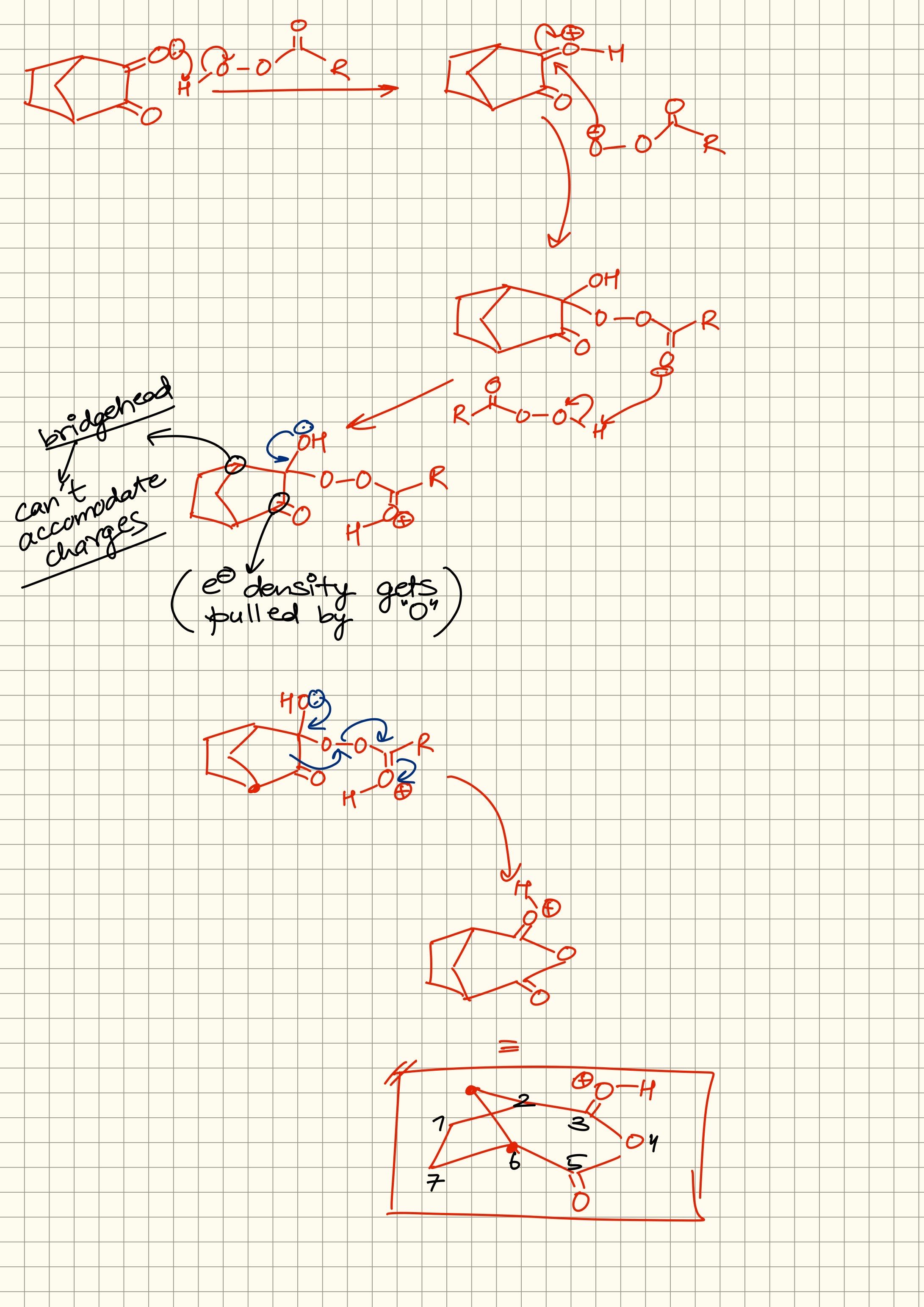
@hardcoreisdead
Inductive to vaise hi 3 carbons ke baad doesn’t affect much
So can’t really comment if it would relatively affect much here
Dono ki apni almost independent electron density hogi
Depend karega situation pe ki kya hone vala hai
As that stack exchange vala answer said
yep
then why is ans AC 😭
Haan to sahi to feel ho Raha hai
6 membered ring
Ohh nahi
Wait wait
aur kitna wait
😭
Yaar lab me hun 😭
oohh tab alag baat hai
PADHAI KARO JAAKE
Padhai nahi
majdoori
Aaj vaise hua yaar kaam thoda thoda
Didi bechaari presentation me busy hain apni 😭
@hardcoreisdead tum bhi padho ye if possible
Mai bhi padhta hun
Fir dekhte hain if we can conclude something
are to khush rho phirr
Haan haan khush hun 😭
Agli internship me karunga iska double kaam
Lad lad ke lunga
hnn better
Yahan thoda shareef ban ne ke chakkar me mai bolta nahi na kuch
😭
Ye bhi for granted le lete fir
😭
I've put this on the chem olympiad server (props to @Opt for suggesting)
Will tell you once I get my discussion over with
alr
some inputs ...

The problem with this though is that epoxidation ka product isn't anyway nearly as stable as baeyer villiger ka product
And the fact that it depends on the situation
(Backed by data)
Proves it furthermore
ughh so confusing
final verdict??
Haven’t made one until now
Trust me, you won’t see this in jee
I’ll still answer you ‘cause I wanna know too