electrochem doubt
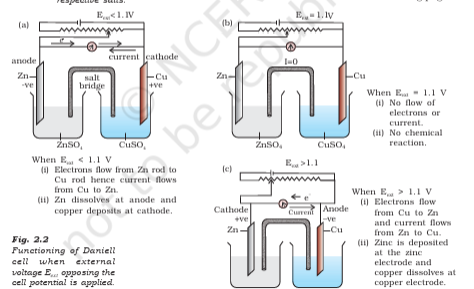
I have trouble understanding this portion. Like I understand the changes that occur as we vary the supply potential as illustrated in this diagram from the NCERT. Why does the switching over of cathode and anode take place though? Like Zn, which was initially the anode, after the supply voltage exceeds 1.1V, starts behaving like a cathode. Why so?
I don't understand the chapter well enough, so please explain in detail.
11 Replies
@Dexter
Note for OP
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.that is because the cathode is not charecterised because of charge but because of the reaction happening there
there is a simple thumb of rule i follow
Jaha Oxidation Waha Anode
Jaha Reduction Waha Cathode
this does not apply for Electrolytic cells tho so keep that in mind waha par cathode is positve only
comng back to here
when the external voltage is less than 1.1V the Cu starts Reducing (Cu ions get deposited to rod after gaining 2 electrons) thus becoming a Cathode
however when it exceeds 1.1V externally we give it enough voltage for Cu ions to lose electrons (which is not spontaneous) and get dissolved
for the Zn electrode which was earlier losing electrons, when given a voltage greater than 1.1V the Zn starts to gain electrons and get deposited
@ns
this is also a good explanation

this explains why electrolytic cells me my funda wont work but its very logical to ratta maarne wala cheez nahi hai
OHHH??? DAMN OKOK
CONCEPT REFINED LESGOO
positive negative is secondary
maing this is red cat an ox
this is the cause
effect is +ve/-ve terminal
okay. thanks a lot
+solved @iTeachChem @Phalawor
Post locked and archived successfully!
Archived by
<@1239101937866248205> (1239101937866248205)
Time
<t:1750834604:R>
Solved by
<@1035556259417571408> (1035556259417571408), <@964432960197632059> (964432960197632059)