QUANTUM NUMBER
Why in unielectron species the energy level of subshells in the same shell are same while they vary in multielectron species? Like why doesn't the energy level depend on the angular quantum number
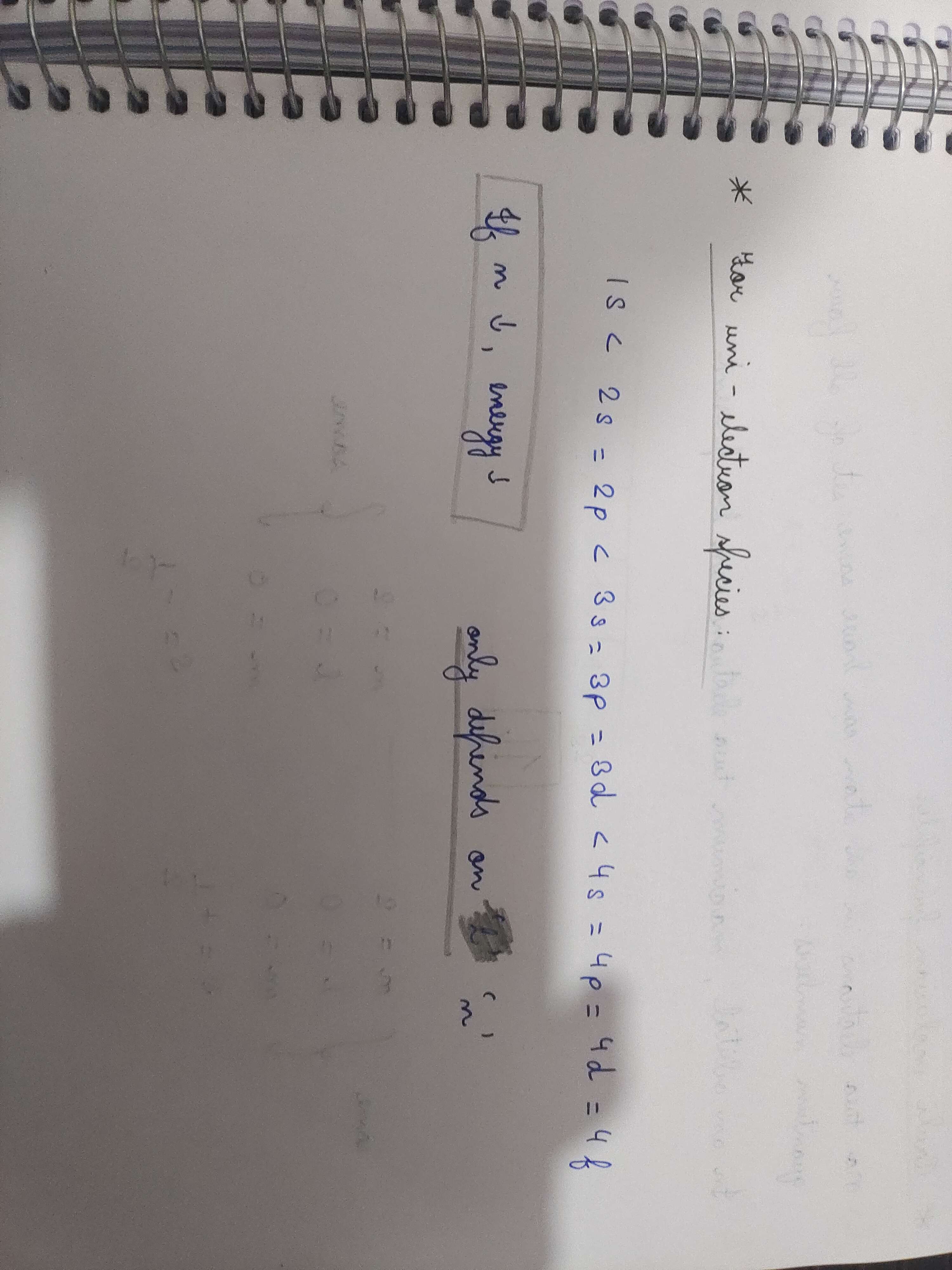
13 Replies
@Dexter
Note for OP
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.Because in multi electron species the electro static force between the nucleus and electron will get distributed unevenly to the different electrons iirc
Like itll pull all the electrons differently
But in single electron species, the pull is only given to one electron, so itll stay same
Only depemding on the radius
So basically a single electron in every orbital = the energy of that particular electron is the energy of the subshell
And I multielectron many electrons are there so it gets filled with many electrons and hence the energy diff
Yep
sahi
Okk
Ji agar samajh gaya hoga toh "+solved @Jeepaglu" kardo 🥹
Bhai ko kitni khushi ho rahi hai
Aye haye
Pehla doubt solved hai ji
Samajhta hun bhai:)
Keep going
Thanku ji
+solved @Jeepaglu
Post locked and archived successfully!
Archived by
<@1051479927008022558> (1051479927008022558)
Time
<t:1750957235:R>
Solved by
<@1386389961196048405> (1386389961196048405)