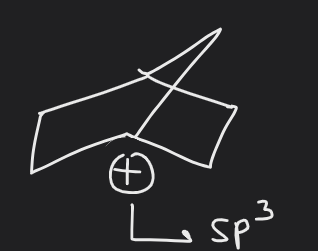
organic- general doubt regarding bridgehead cations
This may be a really dumb doubt but I can't get it. How do I identify the hybridisation of the cation on bridge head? Like in this case no pure p orbital is involved since it's sp3. I've trouble doing this. I understand hybridisation in general but like the cation, anions, and radicals portions just muddles me.
Would appreciate if someone can explain this one along with similar cases for radicals and anions. Bonus doubt: when exactly do we apply bredt's rule? why is that for more than 8 C ring it remains stable?
Would appreciate if someone can explain this one along with similar cases for radicals and anions. Bonus doubt: when exactly do we apply bredt's rule? why is that for more than 8 C ring it remains stable?