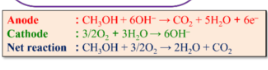
DMFC(methanol burning)
Methanol with O2 rxn ti give co2 and h2o ki agar cell hai to half rxn kaise karenge? aur uski emf kaise karenge?
44 Replies
@Dexter
Note for OP
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.Fuel cell me water is formed :)
Why are you doing this btw? Aise hi outta interest?
https://www.researchgate.net/figure/Schematic-illustration-of-a-PEMFC-b-AMFC-and-c-DMFC-with-half-cell-reaction_fig2_357787762
Idts they'll ask emf without giving reaction potentials
zumdahl
well zumdahl always says just use delta g values
so thats where i am confused
juat gonna paste this here
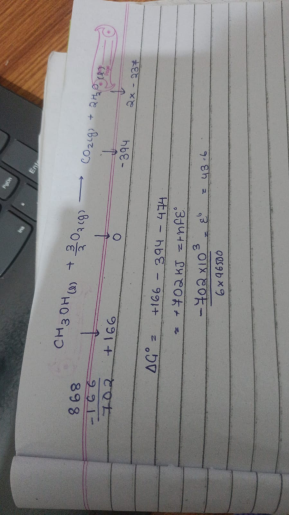
yes thats true
note you only use that cause n is different
G=-nfE
so if you know the cell reaction and electrode potential
hn
using G is correct and it is the actual concept
kyunki G is additive, tum add karke system ka net G nikaal sakte ho
if i made any sense here :')
hnnn
par again hume e not ki jagah del g given hai
coool then, waise here pata bhi nahi n hai kya
of formation of the substance
yes then directly you can find del g of system
agar negative hua
then it is spontaneuous
yh par smh i am getting weird e not
spontaneous
isiliye im like what
matlab tumne kuch calculate kiya hai kya
hnn
e not nikalna hai cell ka
with this rxn
calc bhej do
and im getting weird
wid the question
and data
i was also trying to find the soln to the q real potato sent
maybe im wrong in calc
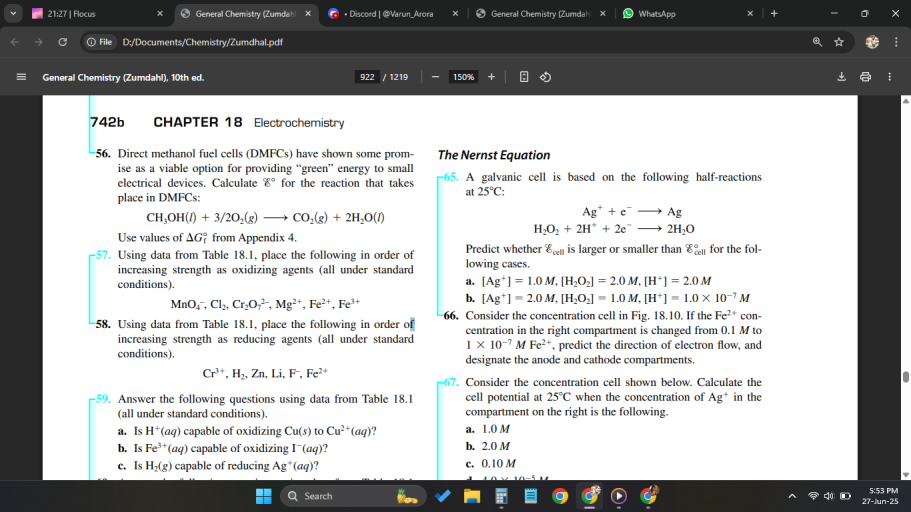
56
ans nahi match kar rha i assume
yeah
-166 - (-394)-(-2*237)
ab try karo
waise delta hum usually final - intial likhte hain
ye pura negative kardena
mai karti ye try abhi mai dusre ke in process hu
accha coolcool
isme n kya hoga?
6 hai shayad
acc to that website
this gives out a VERY LOW E not
what the
0.0017
did you take in kJ
still it is 1.7
if that aint correct then i guess i should retry the question
it is in kj
well zumdahl doesnt give the soln
i meant multiply by 1000
but ah
accha
and i dont even remember what i saw in internet
i'll try to find the source then
yhh
any update?
One is that methanol ka G is prolly -166
Source kahin mila nahi
oh okk
mai dekhti ispe thoda aur

oml
😭 youre right
math
faltu me iska itna stress kiya
+solved @iTeachChem @coolguy.
Post locked and archived successfully!
Archived by
<@888280831863451688> (888280831863451688)
Time
<t:1751913222:R>
Solved by
<@1035556259417571408> (1035556259417571408), <@765933523705921606> (765933523705921606)