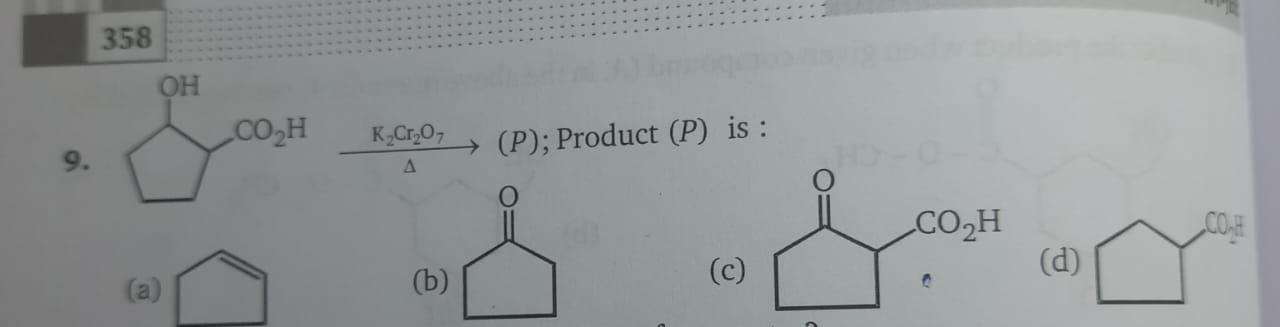
alcohols 2
when we do rxn with k2cr2o7, we make 2 degree to aldehyde. but why should we remove the co2h?
28 Replies
@Dexter
Note for OP
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.strong oxidizing agent hai k2cr2o7
thoda aur explain krd
5 min me karu?
krdo koina
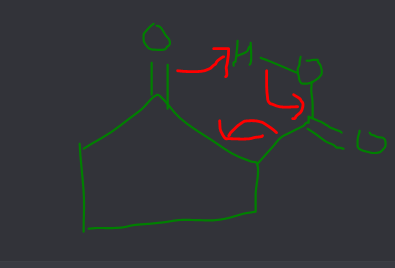
Beta keto acid banega
prabhu agye vese
Usko decarboxylate kar dena
decarboxylate kyun karna ha
Mai library se vapas aa jaaun thoda 😭🙏
Badhiya transition state banti hai
I'll explain
Wait Karo thoda sa
prabhu saambhal lenge
acha koina mai aur post kardu thode doubt
tb tk
Prabhu ki century ho jayegi aaj
100 doubt solved?
Almost
so B ig?
nahi vo to ans theek ha
decarboxylation ki zrurat kya hai
ye puch raha
yeah
beta carboxylic jaldi tuut jata
whaa
ye sb info yaad rkhlu na?
yep
aur ye sab tumhe class mei btaya jata ha kya?
ya khud observe kara ha quesns kr krke?
bataya tha
accha
reason hi dede fir ki kyun jldi tut jata
Beta-keto acids decarboxylate readily due to the stabilization of the resulting carbanion intermediate through resonance with the adjacent ketone group. This stabilization lowers the activation energy of the reaction, making it occur more easily than in other carboxylic acids
👍
+solved @hardcoreisdead @Enamine
Post locked and archived successfully!
Archived by
<@1382187168230936577> (1382187168230936577)
Time
<t:1751909288:R>
Solved by
<@741159941934415883> (741159941934415883), <@984016629119713290> (984016629119713290)