12 Replies
@Dexter
Note for OP
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.The one with the highest oxidation state I guess?
'cause that's the most prone to reduction
So D goes away
As you go down
Size increases
The positive oxidation state they have (of course it's not real charge)
Gets more and more favoured
'cause positive charges spread out better in larger sized atoms
So oxidising power should decrease down the group for the same oxidation state imo
NCERT Padhi?
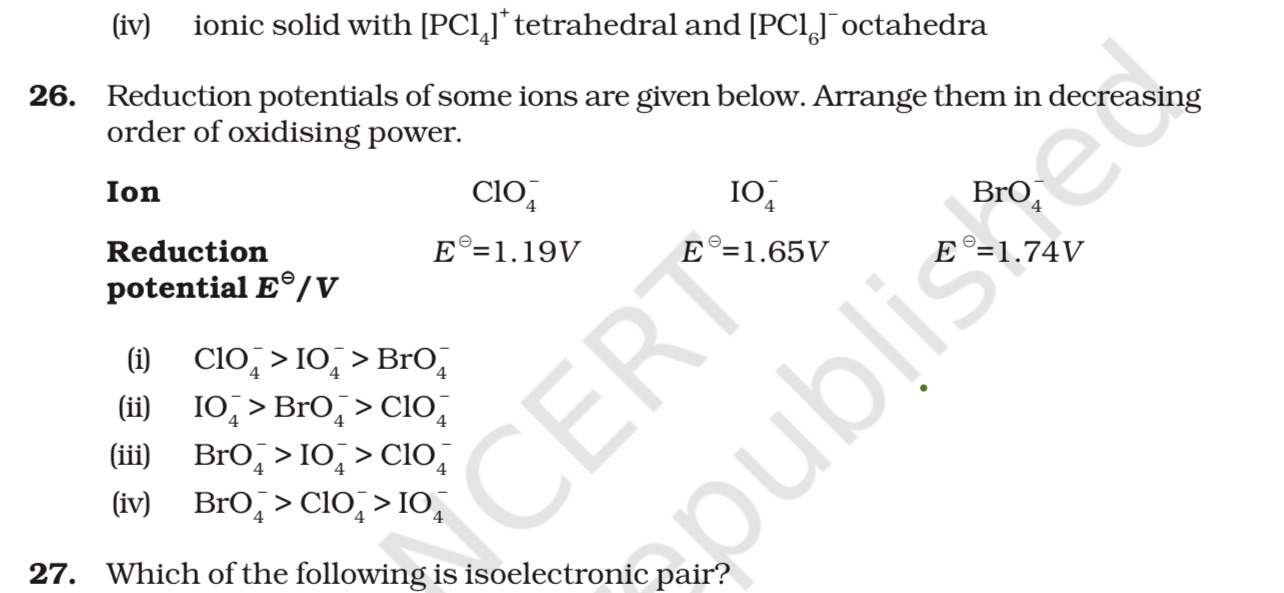
Whattt?
How do you explain this now lol
Exactly that too
And why the sudden shift between BrO4- and IO4-
Kinda trying to find something if I can
I found the exact same question on stack exchange but there's no answer to it
https://chemistry.stackexchange.com/questions/49596/why-are-bromine-oxyanions-uncommon
If someone is interested in knowing why bromine was pushed back
QUESTIONS NAHI KARE AAAAAAAAAAAAAAA
socha module karta hu pehle cuz test is largely based on that
koi ni ;-;
Kind of answers that
Not completely directly
But gives an insight
Exemplar
You either know or you don’t. Such is ioc sadly
no sir bilkul waise nahi liya sir dw
teachers ka kaha kabhi ill not take it that way
its always a positive insight
got it got it ill do that as well
got done with module so thats what ill be doing next (after back exercises ofc)
