39 Replies
@Dexter
Note for OP
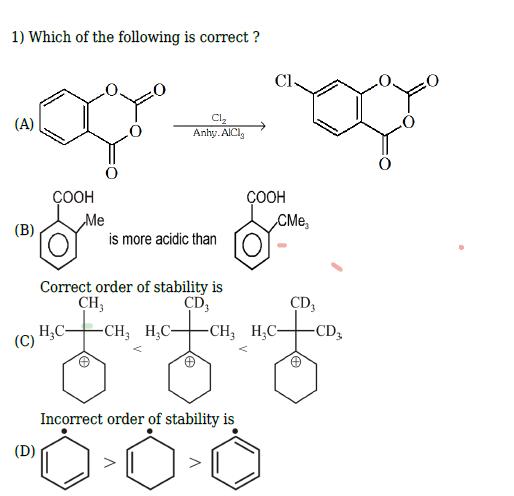
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.deuterium is less stable than hydrogen
it has an extra neutron
D bhi batana ha kya?
but what about +I effect
+H is same in all
well kyunki d me extra neutron hai to thoda alag hoga
im not sure on this but hn alag hona chiye
yeah.. cuz isn't it the correct order? radical on double bond is unstable right.. and first has more conjugation
@Enamine any idea on this
oh okay..
Hmmmmmmm
Ek second
2 sec lelo
Bruh I'm clueless at this one
Kuch khaas difference aayega bhi nahi
and what about opt D?
Option d seems simple enough
Radical on a phenyl
Sp2 pe radical
Not good
(bent's rule)
Yeah so it is the correct order of stability right
the opt says incorrect
Yeah it should be
and the q asks correct
i feel like it's an ans key error
and c should be the right one?
There is something weird about this one
Maybe I'm missing something
There could be an error as well
which one will be more stable in this case? Do we look at the minus charge or the fact that it is aromatic? Like which will dominate
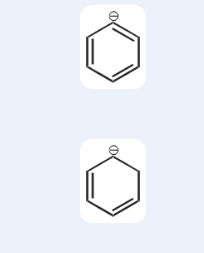
Vo c vaale me to I'm clueless ngl
kyu?
Hyperconjugation ho nahi sakta
aromatic
And inductive vaise bhi itna weak hai
Plus H ka D karne se inductive pe khaas farak nahi padta kuch
but acc to the key the second one's more stable
they said that it's cuz - charge is localised in the first one
Phenyl pe charge something you desire tbh
Yeah
To keep the Aromaticity
It remains charged
yeah plus sp2 pe negative
That charge is very well localised
is good right
so does it become less stable cuz of that
It is but better be delocalised
why dont we look at the fact that it's aromatic
Charge ek jagah rahega to it creates instability
and how do we know which one domiantes
ah okay
See Aromaticity reh Rahi hai
But charge doesn't spread
That's the problem
hmm okay
i get it now
Let me look into that c option wait
Never encountered that not kidding
okay
okay so if there wasnt the unpaired electron we could have easily applied the aromaticity 4n, 4n + 2 rule right?
Bro inductive effect is making me question my existence everyday
Gotta get this shit done fr today