Aromaticity
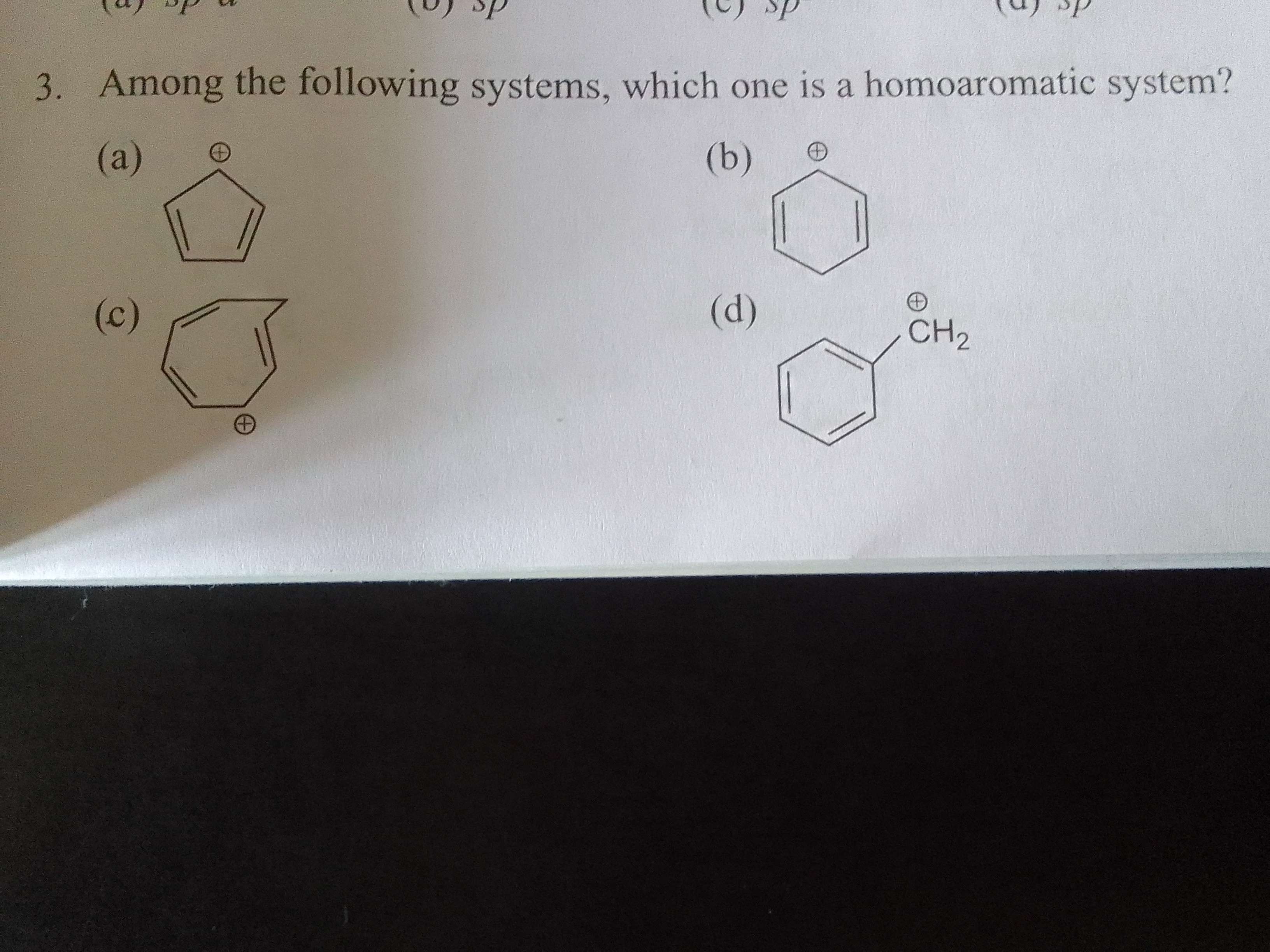
why is the correct answer: (c)? The compound has an sp3 carbon which means it is not planar, goes against the rules of aromaticity, doesn't it?
11 Replies
@Dexter
Note for OP
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.yeah i remember this q (from sykes right)... d seems like the right ans
@Enamine could u pls confirm
i feel c is non aromatic too.. but i dunno

@ns
It's a special case where in continuous ring current and flattened ring causes aromaticity even though a carbon interrupts in between
oh..
i didn't know about this
i thought it's just like aromaticity in general
okay, i got it
thanks so much
Not a problem
It's an overkill for jee anyways dw
Same thing if that sp3 vala carbon is replaced by something else, then it's heteroaromaticity
thank you so much guys 🙏
+solved @ns @Enamine
The bot isn't working so
Won't be marked rn I guess
ohh okay
ah okay.. thnx