33 Replies
@Dexter
Note for OP
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.Who said it's not? 🙂
Btw it's still not as much stabilised though
'cause that pi bond
It resonates inside the ring
Creating aromaticity
aromaticity kese arhi hai? reso kraye to ye to antiaromatic ho jjyega
6 pi electrons na?
Andar bhai ring ke
Ring ke andar
ye to 4 hua na
pi-pi conjugation
2 pi bond 4 electron hi to hua
Vo ring ke just bahar kya hai?
Vo pi bond nahi hai kya?
acha ha
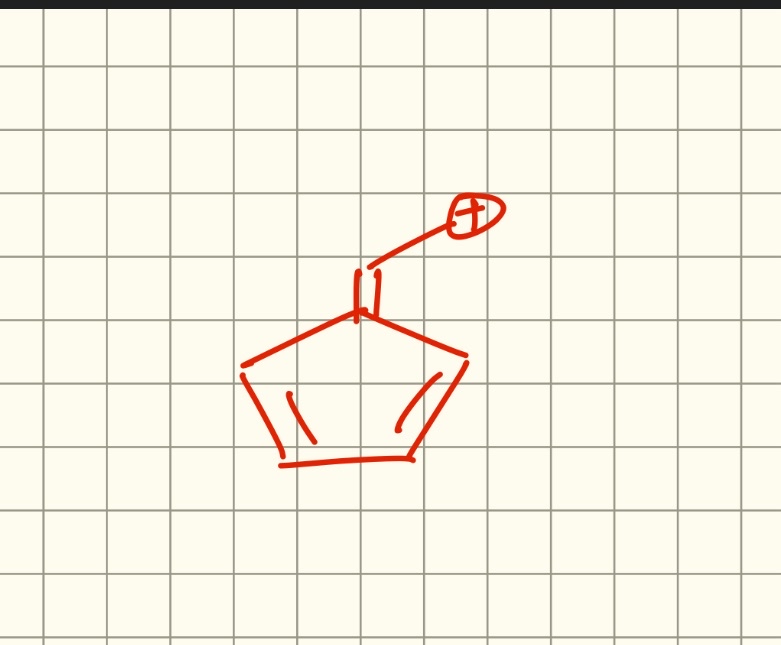
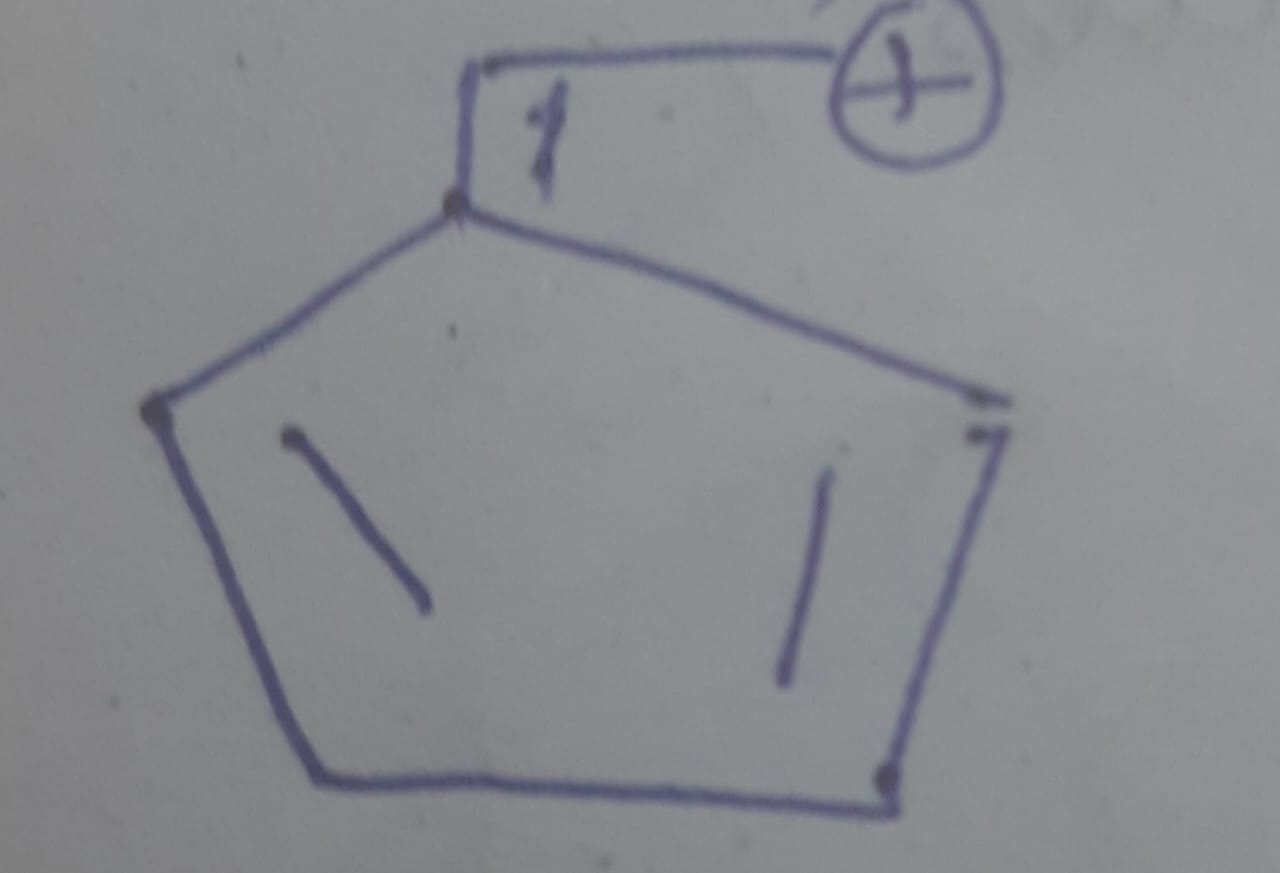
Ye structure hai na?
ring me already ring current tha mene dekha ni mujhe laga non aromatic hai
ha ha right
Tabhi vo positive ki side na jaake ring ke andar jaa raha hai
:sweaty: yus sarr
Sachin Rana [IITB]
YouTube
⭐️Carbocation Rearrangement, Ring Expansion | Aliphatic Hydroca...
Instagram❤️: https://www.instagram.com/iitbsachin
In this video, we are going to understand concepts of Carbocation Rearrangement, Ring Expansion and Contraction, Number of Shifts; also solve problems for JEE Mains and JEE Advance, also we will talk about detailed mechanisms with different questions. A detailed analysis will be done by Sachi...
I explained why
Just above
ye kon h bhai
he said ye resonance me nahi hai
:aah:
yea
so is he wrong or
He's not entirely wrong
Resonance me chala gaya agar
To anti aromatic structure aa raha hai
i dont get this
hm?
6 electtons involved no
That 5 membered ring
Uske ring current me kitne electrons honge
If you draw a resonance structure with that positive charge
Positive aayega ring ke carbon pe?
That would lead to?
4 pi electrons
Anti aromatic
Isiliye vo resonance nahi hoga
i was trying to say this only :haha:
That would be a very minor contributor
wont the double bond out of the ring still contribute to aromaticity
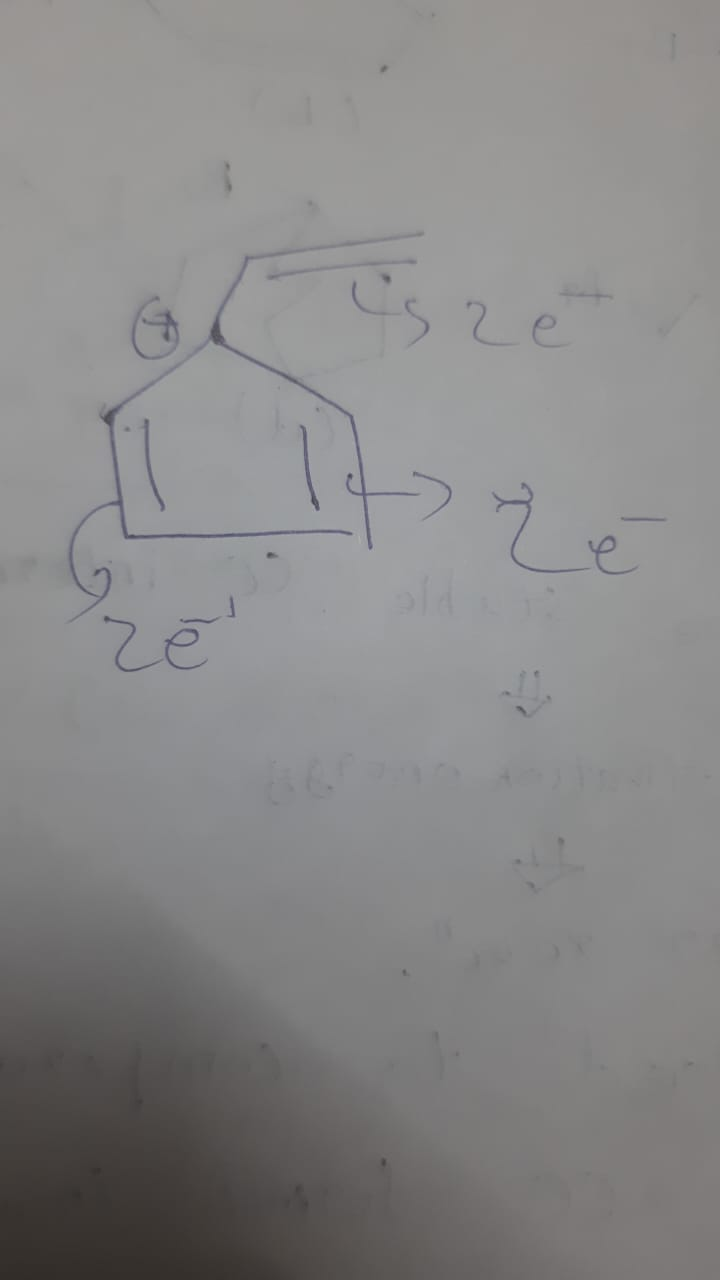
or do i need to look at goc again
It should be conjugated and a part of the ring current
It's not in conjugation with any of the double bonds inside the ring
So it's not a part of the usual 4n+2
it does have pi bonded sp2 orbitals no?
also wdym ring current
The electrons moving around in a continuous fashion inside the ring form the ring current
It is but it's no more interacting with the ring after you draw that resonating structure
Where you take the positive on the ring carbon
why is that tho
sorry if i sound dumb lol
Naah you're not dw
'cause the adjacent p orbitals are the ones that interact
Of course because of the vicinity that they have
Now the adjacent p Orbital to the electrons in the ring doesn't practically have any of the electron density of that pi bond
So they don't count
But
When you had the initial cation
In which the pi bond was just outside the ring
That p orbital had electron density of that pi bond
And could interact with the p orbitals adjacent to it
Do you get it now?
@SirGareth
think so. thank you
+solved @Enamine @Real potato
Post locked and archived successfully!
Archived by
<@856825244728033301> (856825244728033301)
Time
<t:1753042136:R>
Solved by
<@984016629119713290> (984016629119713290), <@1088352651567173632> (1088352651567173632)