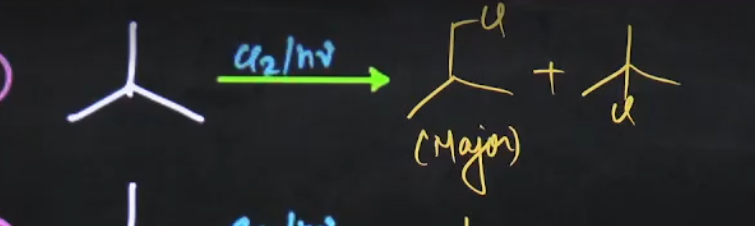
basic hydrocarbons
why this one major product
i knew it yesterday but it just slipped out of my mind today
20 Replies
@Dexter
Note for OP
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.rate hota hai na chlorination bromination me
Usse decide krte hai
1° 2° and 3° ke alag
hindrance maybe?
ye free radicals se banta hai
ab soch ke dekho
Isn't 3 deg radical more stable
That's what I have in mind
Ohh to percentage se find krte hai thanks bro
Selectivity factor
Like 1 degree hydrogens jyada hai
3 degree kam hai thats why?

Yeah kind of but ye photo dekh lo ig smjhoge fir
U can use the rate method too u know in which we say ki rate of 1° position 1 and of 2° is 3.8 and for 3° is 5 to calculate the major product
My bad Bhai ne pehle hi bata diya
free radical mechanism.
Sir lekin free radical mechanism mai more stable radical hi toh banega Hume yaha exact rate compare karni chahiye na ? Or they both are same methods ?
i will bow out of this since i am oc noob :P what @Real potato said, lets go with that
@Carnot Engine
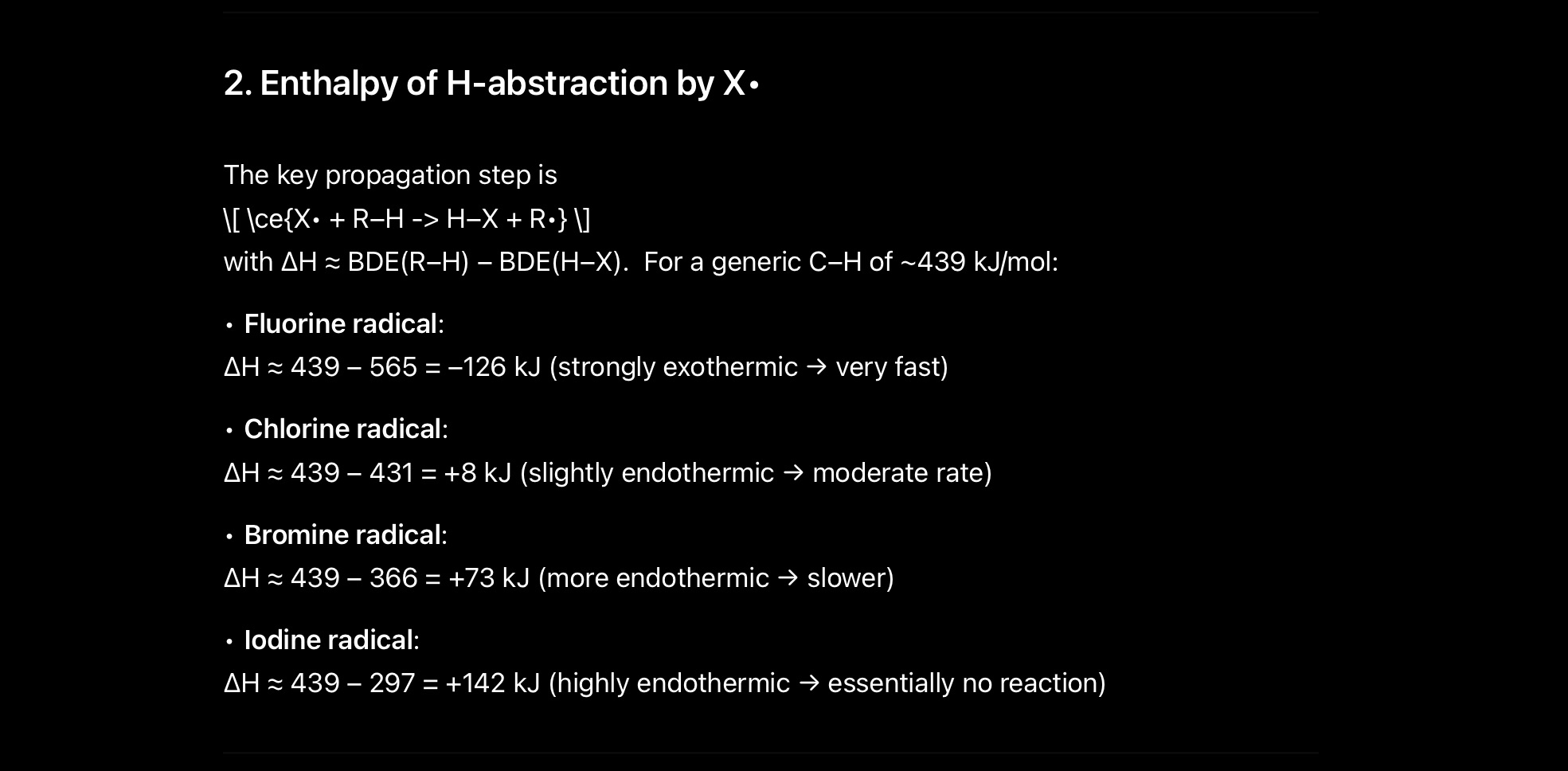
Check out this data
There are 3 steps na?
Of the whole reaction
First one is just X-X bond cleavage
That’s not really critical
Why?
‘cause you’re providing sunlight
Acchi khaasi energy mil jayegi
Then after that
The propagation step is what determines how will the reaction go and which H will be abstracted
Ab Jo us step ka energy change hai
It’s moderate for Cl2
Not very high
Not very low
Now what that does is, statistics aa jaati hai bich me
Jitne jyada 2 degree ke positions honge
More likely for the Cl radical to interact with them
Because of these two competing factors, we see that gradient
Which @Real potato mentioned above
So yes
You gotta remember the data
But
Now you know the reason why
And if you wanna ask why bond energies are like that….
That’s entirely a different story
Hmmm nice thanks 👍
Ha yahi 👍🏿
+solved @Carnot Engine
Post locked and archived successfully!
Archived by
<@1179817028106858538> (1179817028106858538)
Time
<t:1753241825:R>
Solved by
<@797745662497652746> (797745662497652746)