org chem mixed
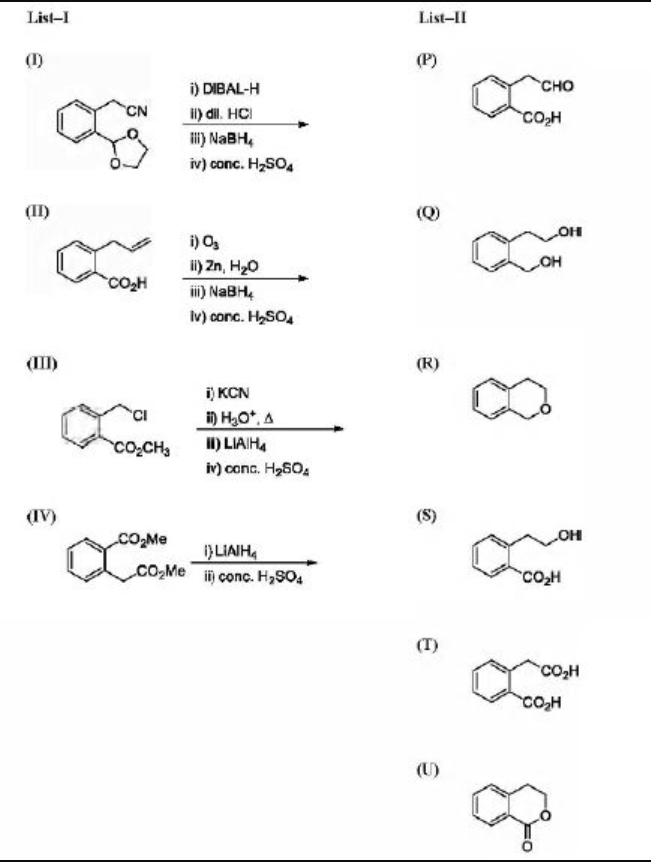
List-I includes starting materials and reagents of selected chemical reactions. List-II gives structures of compounds that may be formed as intermediate products and/or final products from the reactions of List-I.
68 Replies
@Dexter
Note for OP
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.wont S be formed in part 3 anywhere
judging by the options one oh is removed leaving a carbocation on which lone pair of other oh attacks
@Enamine
SN2 boi
KCN does SN2 on that benzylic position
saare parts discuss kare ek ek karke?
ping me if yes
Bas 2 min
Coming
alr
@Enamine
🥀
koina yar🥀
Guys
It was something important
Had to go
Then I slept
Sorry for that
I just woke up
Will get to this
Yeah yea
Np take your time
Hello
@hardcoreisdead
You there rn
We can get this done then
I am in class rn
In 4th part both the esters will be attacked simultaneously right
@Enamine aa jao sir ji
Aa gaya ji
Haan
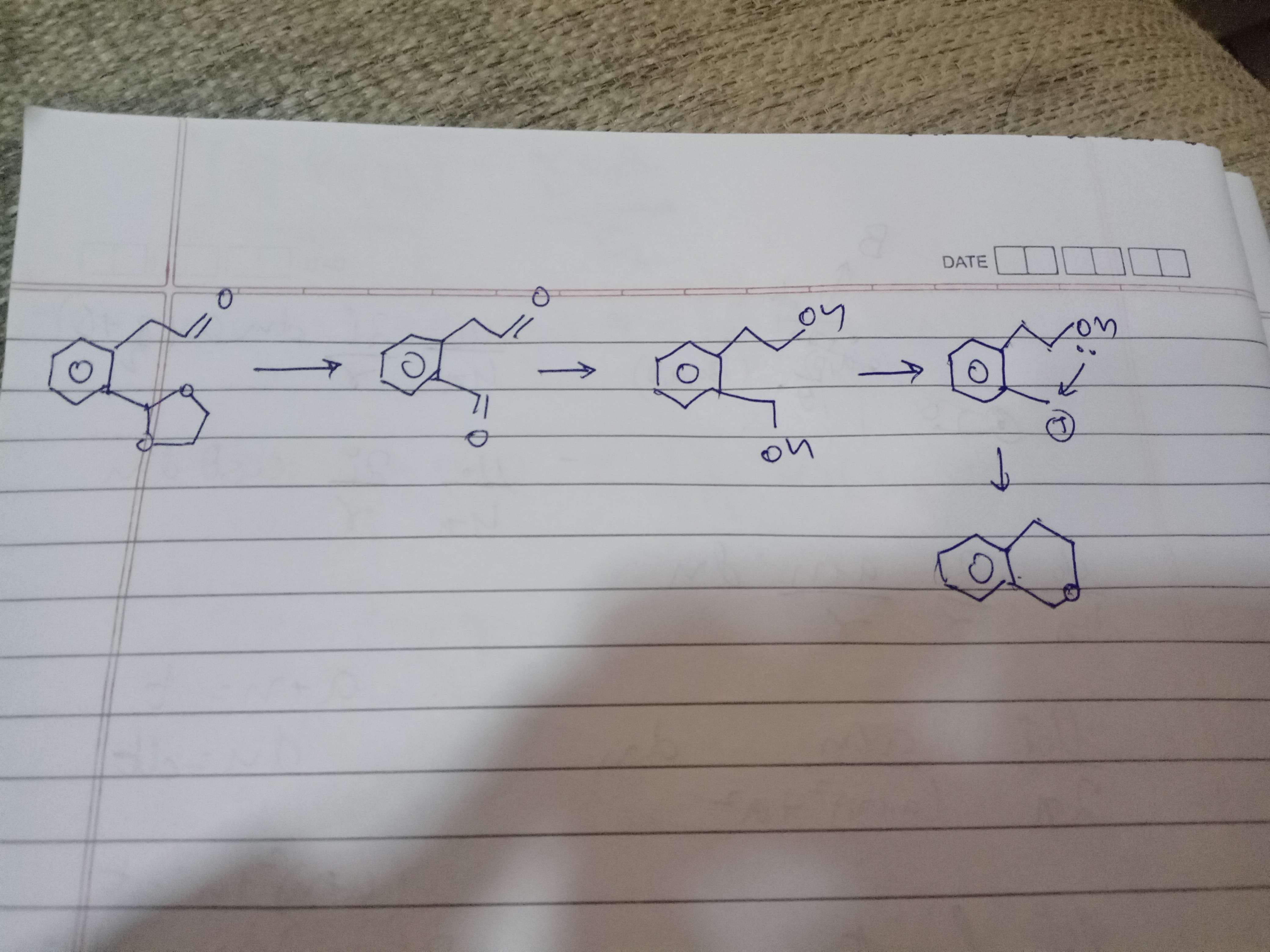
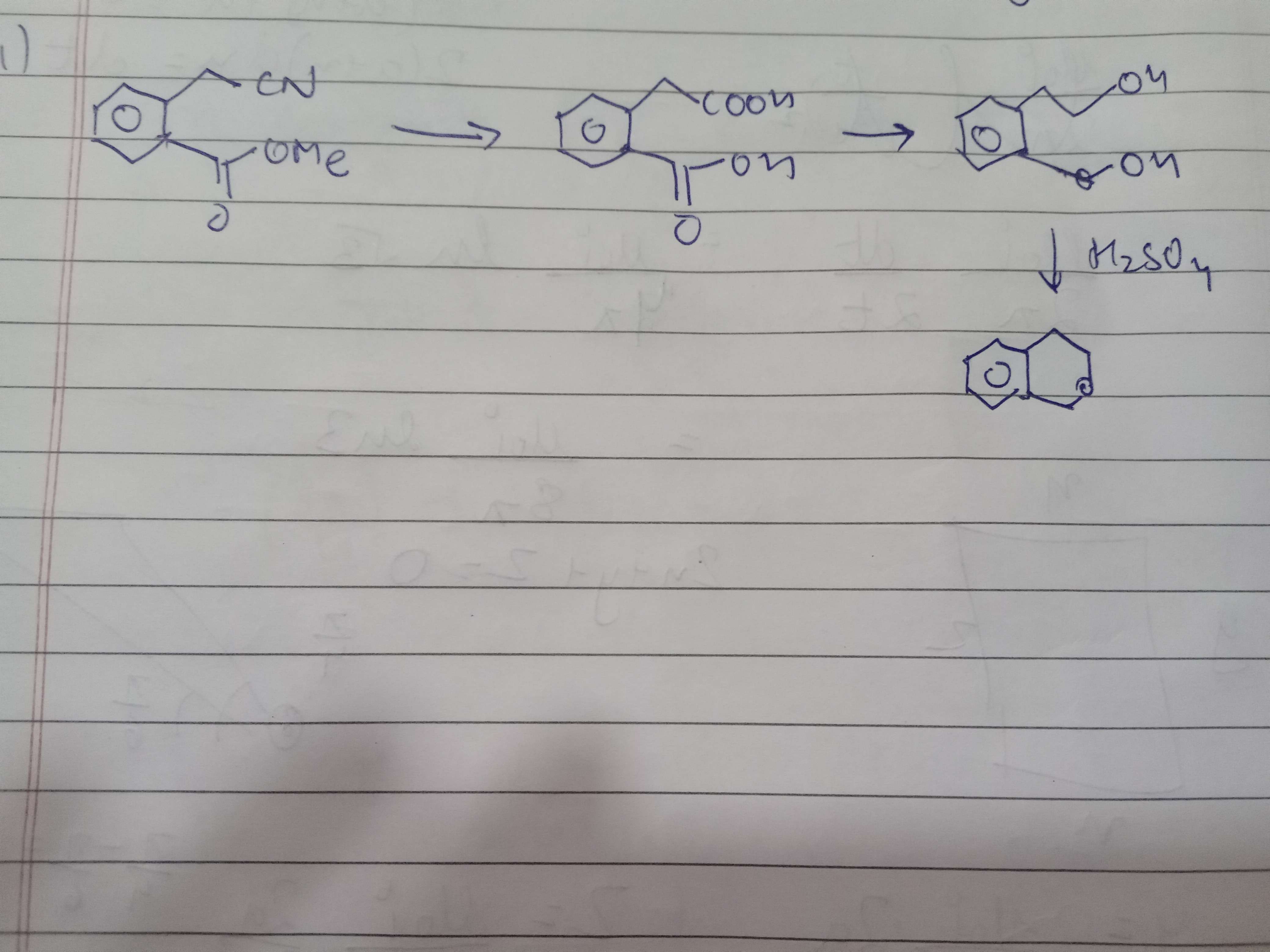
To first part se
First se cyanide ka aldehyde
Second step me hydrolysis se acetal ka aldehyde
Third step me aldehydes ka reduction
Fourth step is dehydration and formation of 6 membered ring
So R banega
multi match ...
This is the 1st one
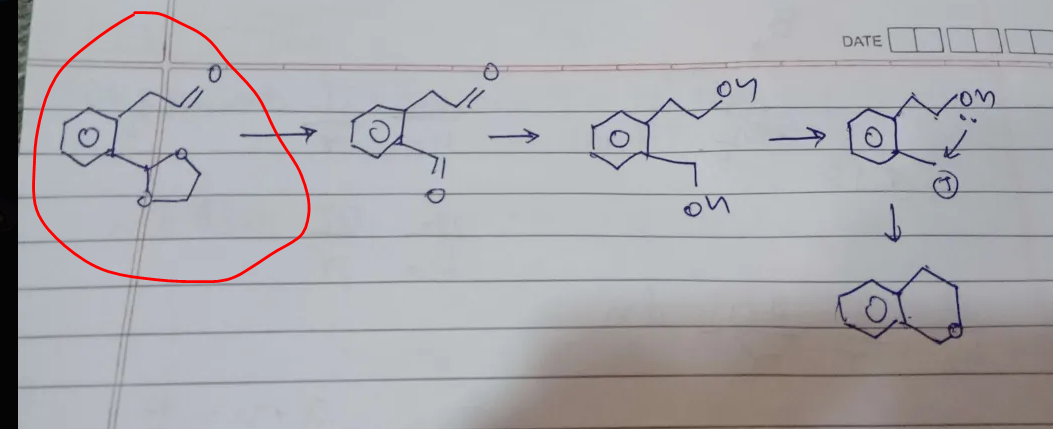
now -
1. why does only one -oh is protonated and removed
2. why is it the lower one
acc to me-
1. ring formation possible
2. much more stable carbocation
Your reasoning seems about right
Major driving force is the formation of the 6 membered ring though
As one of your reasons states
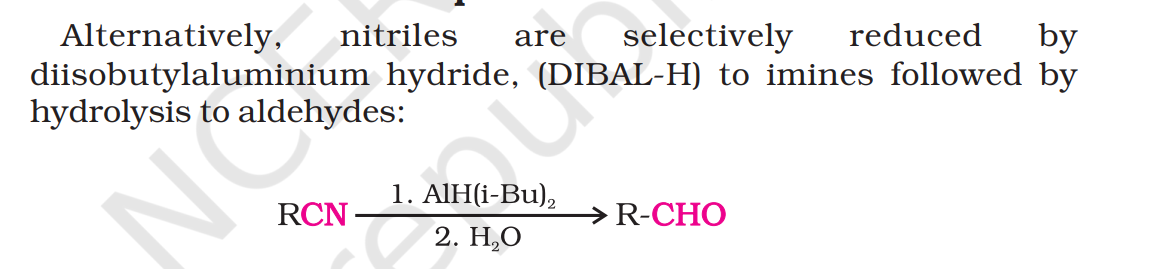
but in 1 shouldnt its be imine because dibal h is given but no hydrolysis
No problem in 2nd ig
dil hcl
essentially h3o+
accha uske baad se likha hai then all good
actually meine thoda galat hi likha hai
sirf dibal h daalke kya banta 1st mein
ig imine
dil hcl will make aldehyde and will also make aldehyde from the ethylene glycol thing
DIBAL ke saath work up is almost always necessary 'cause aage kaam hi nahi ho payega kuch
Reaction is occurring at -78°C
That complex would break apart
ohk
accha haan right
Yo @hardcoreisdead
You there right?
hnn
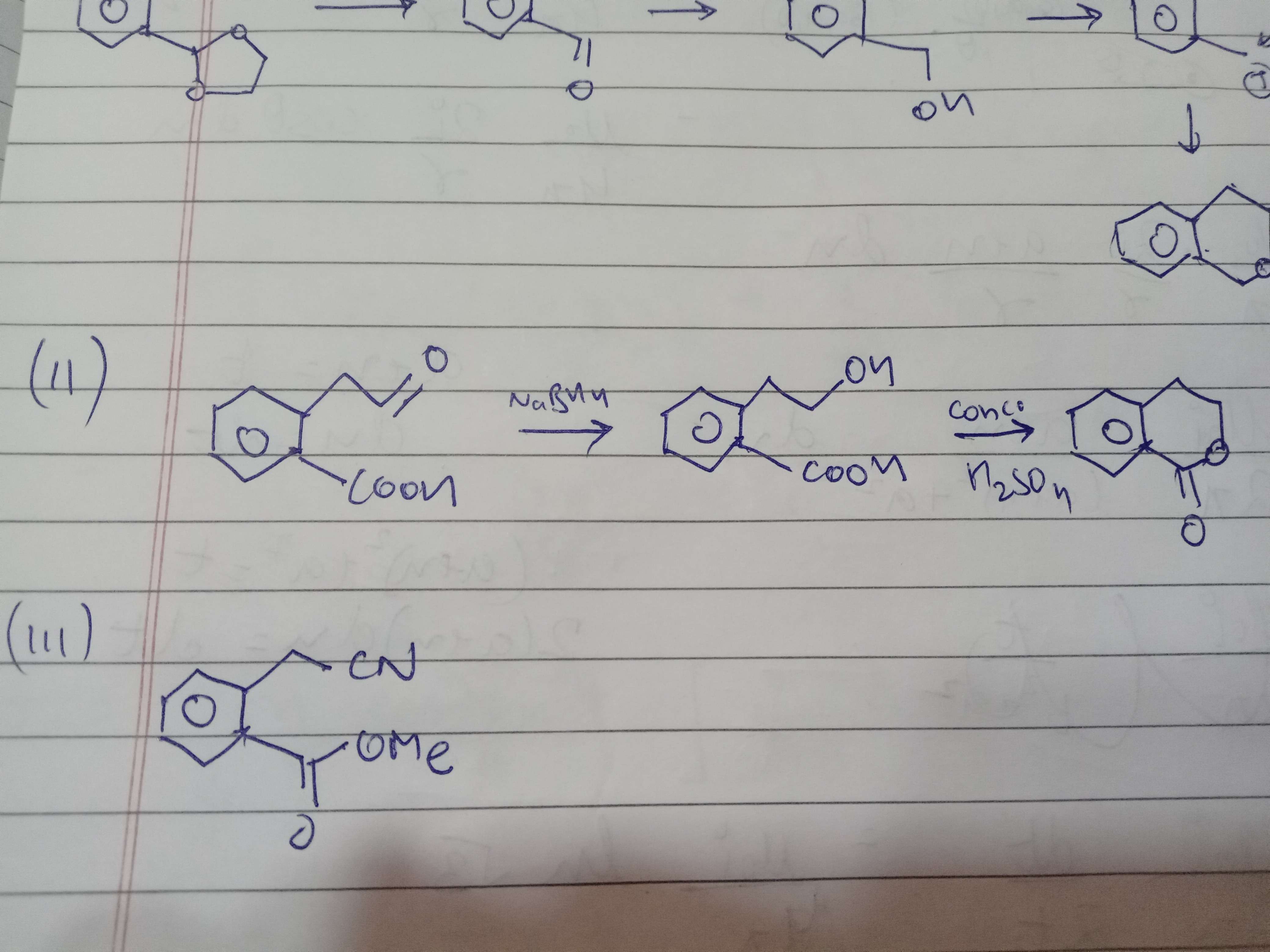
Second me
Deductive Ozonolysis so you get an aldehyde over there
Fir reduction okay
it always is but for the sake of it
(i) ke baad kya hoga
Hmm still it's give anyways
Konsa (i)?
1st wale mein (i) ke baad ka product
basically what we are discussing
Hydrolysis kardo poora
achaaa
Aldehyde over there
Upar vaale pe
Niche be aldehyde
Like baad me HCl daal diya na dilute as you said
so the red one wont be formed
uske next wle se hi baat karenge
Nope
neeche me aldehyde ii ke baad banega right?
yep
First ke baad I suppose iminium rahega
Then afterwards
Hydrolysis
Usse dono aldehyde
Clear with this one now?
This for the 3rd
1. Why doesn't cn- does nucleophilic attack and form cyanohydrin
yep
The C=O is not electrophilic enough
Ester hai
Not aldehyde or ketone
And also
You need CN-
K+ CN-
bro i still think ii ke baad banna chahie uppar me aldehyde
Hmm accha haan alright
waise toh it doesnt matter ultimately banega wahi
han whi toh bola
like hydrolysis ke baad hi banega
Haan bich me iminium hoga
rxn i in itself has no significance without workup
hmm ok
right
So
@hardcoreisdead
Ester pe nahi ho sakta attack
also why cant i remove ome- and put cn- in its place
Uske liye attack karna padega na
That is the whole point
Agar laga diya
i get nucleophilic nahi hoga cuz very less electrophilic
To OMe- attacks again
but substitution kyu nahi
Substitution kahan kara rahe ho draw karke bhejo
MeOH banke nikal jayega na after next step
Draw karke bhejo I'm not getting your point
nvm sp2 pe substitution kara rha tha 💀
.......
Mai vahi sochun
Ki kya bole Jaa Raha hai
I was like itna dumb to nahi keh raha hoga kuch 😭🙏
You surpassed my expectation 😭
(sorry happens dw)
lol 💀
In 4th part both the esters will be attacked simultaneously right
Yeah yeah
Too much LiAlH4 for them lol
alr
marking it solved
+solved @Enamine
Post locked and archived successfully!
Archived by
<@741159941934415883> (741159941934415883)
Time
<t:1753539640:R>
Solved by
<@984016629119713290> (984016629119713290)