20 Replies
@Dexter
Note for OP
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.B toh nahi hoga ans. Itna simple nahi ho sakta
Bhai close mat karna pls. Mein baadmein dekhunga
@Prasan @hardcoreisdead

yea yea
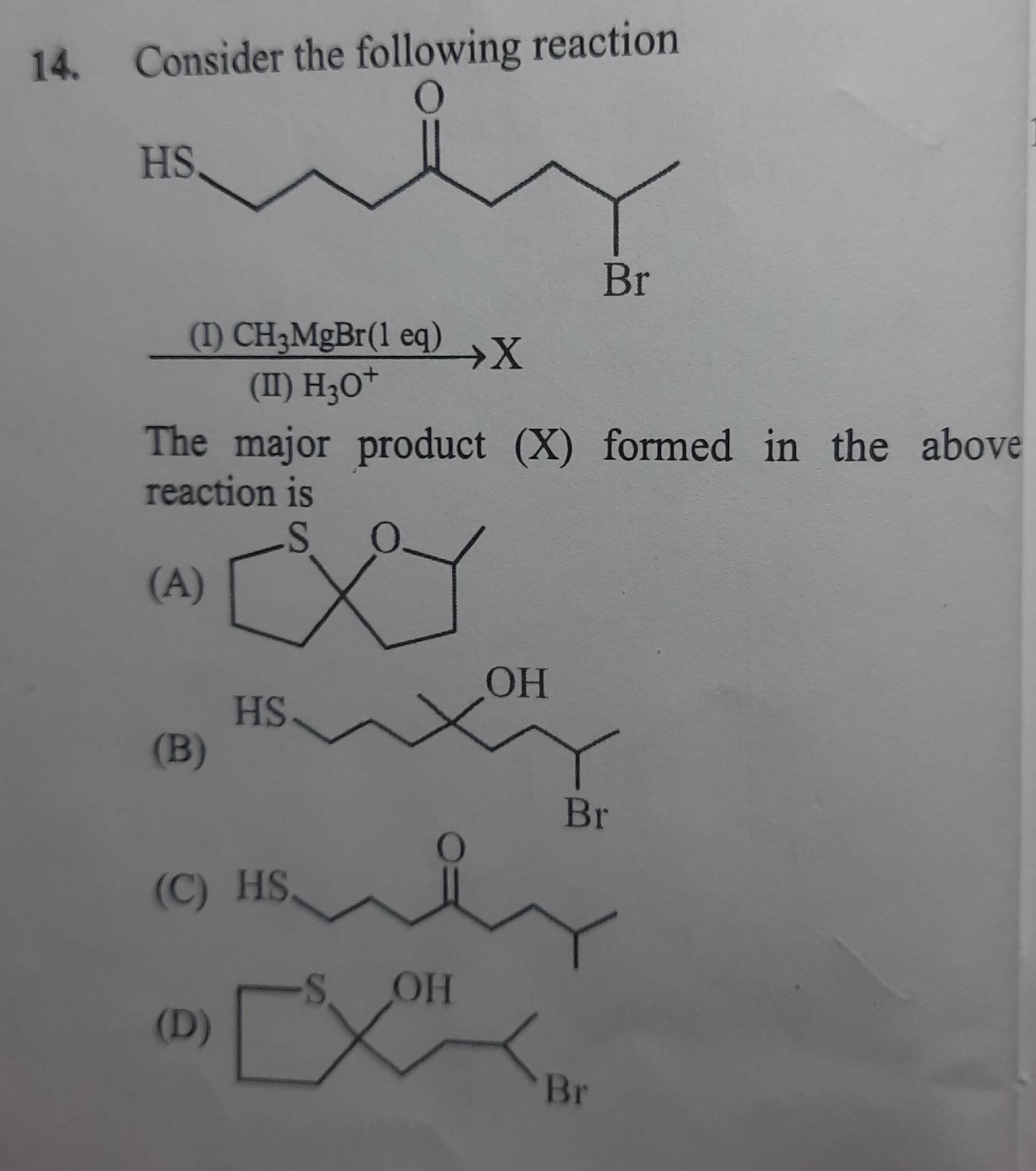
why isnt B option feasible
How is the red part a 3 member ring
C-SH> c=0 > c-br
Order of electrophilicity??
Could be, S pe density zyada ha
Sir that's 8
Naah
C=O is maximum
Reason kya ha iska
Electronegativity difference or 2 bonds hona
2 bonds me se electrons pull kar raha hai oxygen
Acha
why did u extract H from SH but not from the carbon besides c=o
they are way more acidic
Thiols are more acidic buddy kya bol rahe
@hardcoreisdead
Oxygen ke group me hai sulphur
Uske saath laga hai hydrogen
Usse jyada acidic kaise hoga carbon ka H
even if H+ nikalke -ve charge resonate kare with c=o
Sulphur ka size to dekho
3rd period
A whole freakin shell added
Better stability of negative
convincing
grignard acts as base and removes the acidic H
and then just ring formation
Yes
@hardcoreisdead
alr
@Prasan mark solved
+solved @Varun_Arora
Post locked and archived successfully!
Archived by
<@1382187168230936577> (1382187168230936577)
Time
<t:1753637608:R>
Solved by
<@984016629119713290> (984016629119713290)