116 Replies
@Dexter
Note for OP
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.last one
uhh
sn2 me non polar solvent ke hisab se dekhna hai shayad?
hmm maybe not
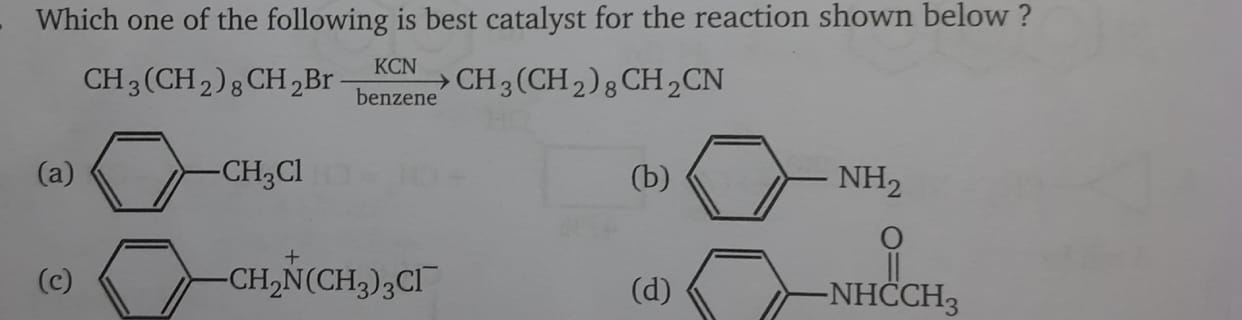
sn2 hogi?
solvent beneze hai
nahi shayad
hmm vahi
no kis karan se
mai puchra tha tune bola na upar
sn2 hogi yes
ambident nucleophile
acha
tbbhi fir kya akrein
A nahi hai fs
baaki nhi samjh aa rha
prabhu ki zarurat ha
aisa prateet hota hai ki mano prabhu vishram kar rhe hai
let the soldier rest for a while
satya ha
Prabhu vishram nahi kar raha ab Prabhu ke paas samay nahi hai 🙂
ARE PRABHU
Subah na nahana pade isiliye abhi nahane Gaya tha
pranam
Or kal subah 9 se 1 class hai
Fir dopahar ko 2 se 5 baje tak lab session hai
Poora din is packed af
And maths thodi bhayanak ho gayi hai 🙂
koi baat nahi prabhu, aaj aapke shishyon nei khud ke saval khud hi bana dale
So ab mujhe thoda roj padhna padega 😭🙏
bas ek saval rehgaya ha
Yahi vala na?
yeah
Ruko ye to dekh hi leta hun
Dekho Jo mujhe lag raha hai
First glance
Br- jiske saath attach ho jaaye
why so
isme dekhna kya ha exactly
Dekho
Rxb ho gayi
Uske baad Br- nikla hai
arey paay laagu
So agar vo kahin or chala jaaye reaction ke bahar
bro lives in future
Bas karo lmao 😭🙏
So le chatelier principle kinda
That takes the reaction more forward
lechatelier principle idhar
Although this is not reversible
Basically tum right side se kuch hatayoge
To left to right jayegi na reaction jyada
You have the intuition for that from equilibrium and thermo right?
haan haan
kahin bhi lag sakta woh
So Br- ko hatana hai
Somehow
especially jab kinetics ki baat ho
So B and D na ho rahe
B khud nucleophilic hai
hmm dhyan mein nahi aaya tha ye
Won't interact with Br-
okayy
D bhi nahi kar payega
Amide hai
It isn't electrophilic at all
Requires very strong nucleophile
So it's either A or C
Br nahi kar payega vo
Yes
So now
I'm leaning more towards A
'cause Br- vahan consume hone lagega
But again
You see Cl- forming there
Which can attack again
And form the catalyst again
a mein?
So thoda doubtful
Yes
hmm answer c ha vese
ek sec how do we want the solvent to be
polar aprotic right?
SN2 hai to polar aprotic
def not. KCN uske saath react kar jayega
I don't have a very good reason but mai C ke hi saath jaata
Yeah a good enough reason
ek sec solvent benzene nahi ha
So basically all we are trying to do is
Keeping Br- busy
So at some instant
The equilibrium shifts forward
Not in a very significant amount
But it does
Yes but as I've told you guys before solvent isn't a requirement
It's a perk
It facilitates the reaction
Never decides the mechanism
Isn't
*
agar ek solvent acidic ha aur ek basic to vo mech decide nahi karte?
Mai tab baat kar raha hun jab solvent react nahi kar raha actually
acha vo theek ha
Acidic basic ke case me to solvent khud reagent hai na
yea ya
So C ke case me
Vo Cl- ko replace karta rahega
From time to time
Kabhi Br-
Kabhi Cl-
sorry i missed the above part can u explain B and D kyu nahi hai
Then after a while jiski lattice enthalpy jyada hogi
Vo stabilise ho jayega but hamara kaam ho jayega tab tak
i figure aniline is a good nucleophile so cn ki jagah badi chain uspe jayegi ??
bit confused tbh
B is nucleophilic itself so won't budge with Br-
D is an amide; not anywhere near electrophilic so Br- won't interact
Cyanides aren't very electrophilic to break it to you:)
Sadly it's taught this way ki cyanide pe attack ho gaya
But unless the medium is acidic
can we say that due to resonance theres high electron density on oxygen which acts as nucleophile
Cyanides don't behave as electrophiles
Which oxygen are you talking about
unpe toh waise bhi -ve chrge hai electrophile toh ban hi ni sakta
in D
C=0 one
vo double bond vala O
Just understand ki Br- Cl- exchange hote rehenge
That oxygen is sp2:)
Not very nucleophilic
hmm kabhi ek lagke tut gaya, kabhi dusra
Chahe electron density ho bhi
resonance toh ho rhi na
N ke lp
resonate karenge
aisa kyun hota ha
toh Oxygen pe -ve ayaega na
haan sahi baat ha
But neutral resonance structure jyada major hogi
Ek counter question hai mera
When you study enolates
Why do you attack from the carbon
yeh baat bhi shi hai
Why not oxygen?
hmm
Negative charge aa raha hai uspe
You see the problem is
You have a better and more nucleophilic thing to attack
O ka -ve neeche laake double bond se karte right
So statistically favour hoga
haan
Ki better chiz attack kare
Yeah that but attack carbon ke negative se hota hai na
That's what we are concerned about rn
han
Vaise hi
Is case me bhi
acha isliye O ko chod dete aise hi
Nitrogen is more nucleophilic than that oxygen
In the same molecule
yeah thats true
Yup
Uska attack hoga bhi
To that would recerse
Reverse*
acha B aur D to sorted ha fir
Yup B and D nope
A as hardcore said
React kar lega
That CN will react
So we are left with C
And that's correct:)
ek bar
A react kyu karega reason dedo
bhai abhi band mat karna
ill read this again later
mera ha doubt abhi
acha theek h
A is benzylic right
yep
SN2 acchi hoti hai benzylic pe
Padha hoga
haan
Iska reason is in molecular orbitals
To thoda mushkil hoga
Samjhana
reason chodo abhi
Hmm
itna hi bahut ha
To benzylic position pe CN react karke
Reagent consume ho jayega
Hamara actual molecule react karne ki bajaye benzylic vala karne lag jayega
alrightt
ho gaya lagta
open rkhta kl firse pdhunga
Kuch ho to pooch lo
Mai bas jaake sota hun
aap vishraam kariye prabhu
Wish me luck
Kal mai nahi meri laash vapas aayegi classes ke baad 😭🙏
prabhu ko kon hara sakta ha
Bhai itna hectic pichle 1 saal me kabhi nahi hua jitna kal hai
Bhai tumne bohot sir chadha rakha hai mujhe 😭🙏
sbke saath hota koina
Hmm vo to hai
Chalo jaata hun
Bye
byee
Kardun close? @hardcoreisdead