36 Replies
@Dexter
Note for OP
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.b if i remember right
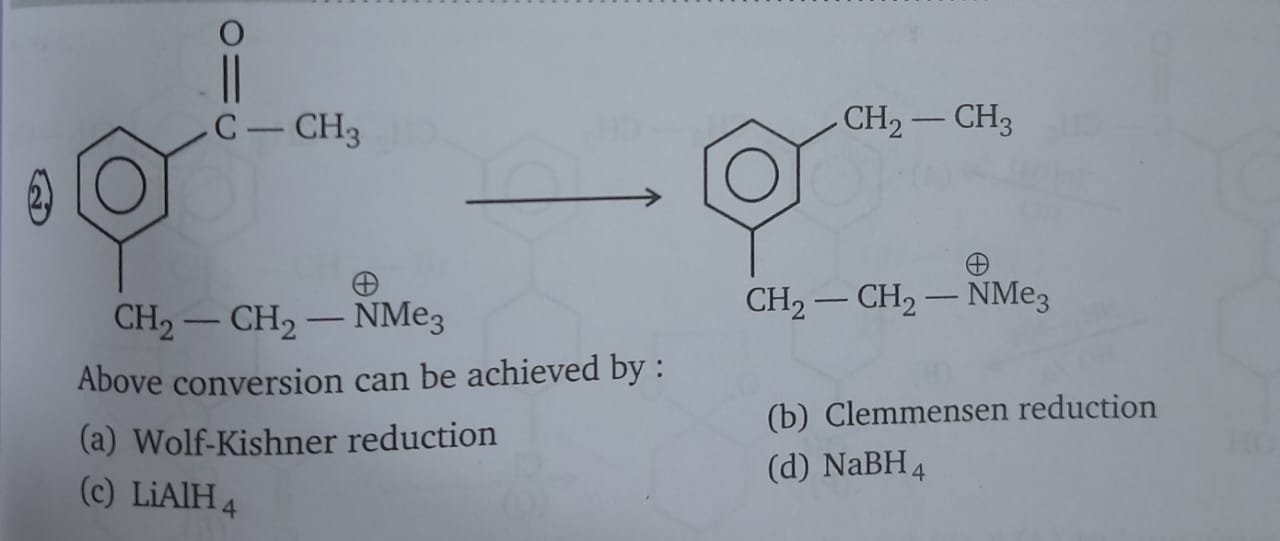
isme ald aur ketone ko alkane bana dete hai
zn aur kuch to daalke
ik min notes check karke batati
yes
to ZnHg and HCl use karke ald aur ketone ko alkane bana dete hai
aur iska name hai clemmensen reduction
par
vo to baakion mein bhi nahi hota
Hn to it is asking kisme hoga na
Baki me kya product banega nikal ke dekho
lialh4 aagar daala to kya ayega etc etc
is it multicorrect?
or single correct
ig both a and b should be it
lialh4 and nabh4 are to synthesise alkanes from alcohols.
yea vahi mai kehraha but ans b ha
isliye to doubt pucha tha
oh okay
hmm im not sure why wolff kishner wont work here
pretty much the same process
yeah
prabhu chahiye abto
maybe has got to do smtg with the nme3+
maybe wolf kishner mein acid base ho jaye
nh2-nh2 is a base
hmm ho sakta ha
acid kya lega
acid to ha ni sahi baat ha
is the H here acidic enough?
try to karo aur apna attempt bhejo ik vaar uspe help karenge yaha
Dekho wolff-kishner kya hai
Its N2H4/KOH and ethylene glycol
Ab dekho reactant mei kya hai
It's NMe3+ aur wolf-kishner mei OH- hai
To ye elimination of tetra alkyl ammonium salt ka case ho gaya
To product mei carbonyl ko reduce karne ke saath-saath NMe3+ most acidic beta-H ke saath bahar nikalkar double bond banayega to question mei ye wala product nahi banega
oh yeah
hoffmann exhaustive methylation
lemme see
big brain
it's basically that case where the hoffman elimination product is more stable due to -I of nme3+
achaa
acha yaad agya
vo base bada hota jab
tb hoffmann vala lete
ha that's for tbutyl
but ha usme 3-4 cases the
koina base bada chahiye ye vala bhi bada hi ha
one among that
whats the problem with lialh4
and nabh4
Carbonyl ko 2 degree alcohol mei convert kar deta hai
You will study in Alcohol, Phenol and Ether
To vo to pehle hi rule out hogaya
aur nabh4?
Vo bhi same hi karta hai
Usko 2 degree alcohol mei convert karega
omg why tf did i think double bond gayab ho jayega
oh shit
Grignard reagent padh liya ??
Usmei bhi same concept hai
theek ha mai smjh gaya
+solved @Prachi
Post locked and archived successfully!
Archived by
<@1382187168230936577> (1382187168230936577)
Time
<t:1754415899:R>
Solved by
<@926887811674673172> (926887811674673172)