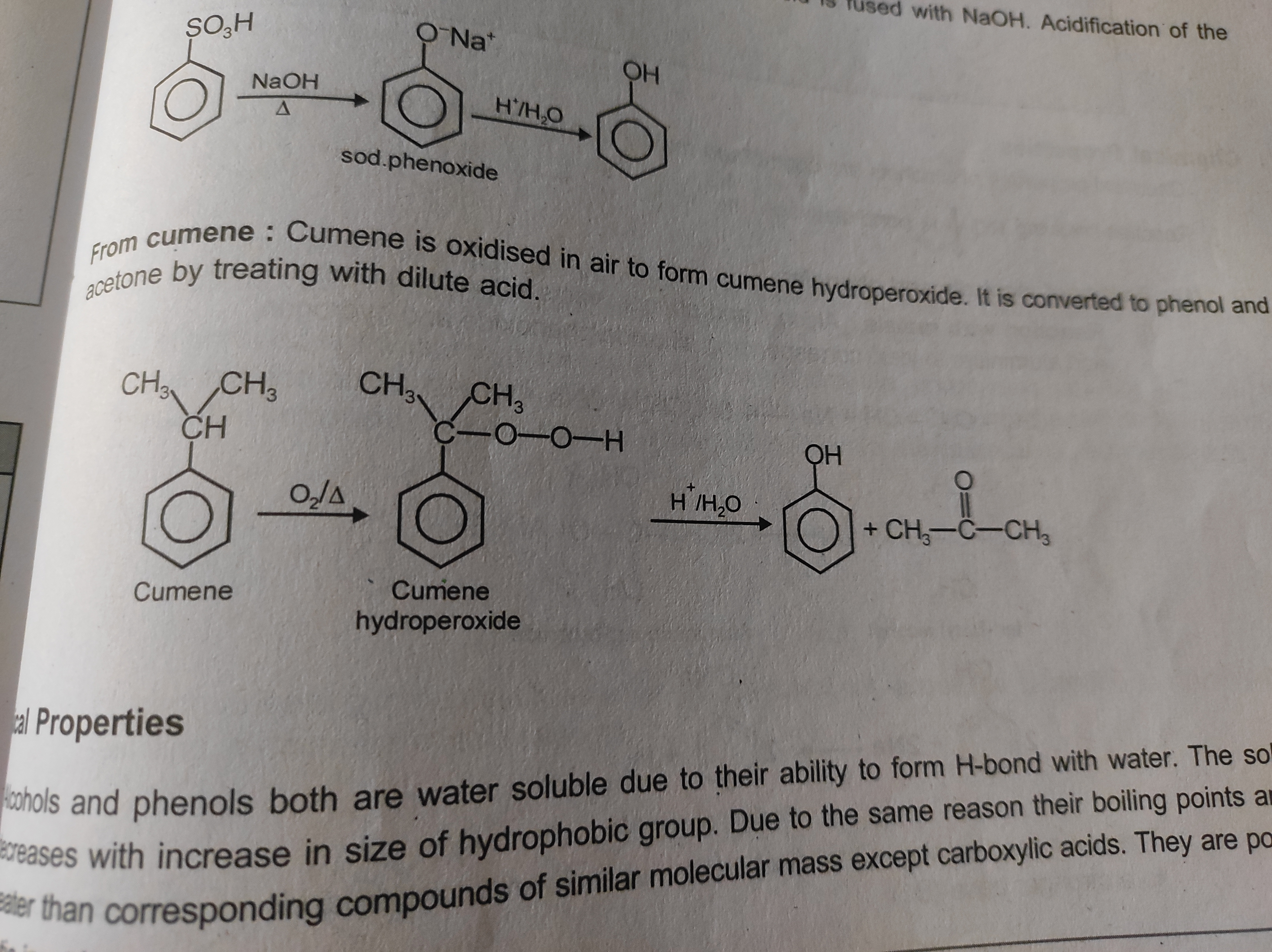
Cumene to phenol
why and how does the oxygen get bonded here to benzene? I looked up the mechanism but it didnt made much sense of how the product is going to be rearranged that way
26 Replies
@Dexter
Note for OP
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.Oh this is cumene hydroperoxide rxn
i hve the mechanism wait ill share
yp tht one
don't understand how the mech works
like why the oxygen shifts
Pehle jo OH hai vo H+ par attack karke use OH2+ banayega jo ki ek good leaving group hai
Aur jaise ham carbocation rearrangement mei karte hai 1,2 shifting vaise hi isme phenyl ki shifting ho jaati hai aur H2O bahar nikal jata hai
par vo phenyl shift kyu hoga? Mujhe vo nhi dikh rha
sry thoda sa messy hai
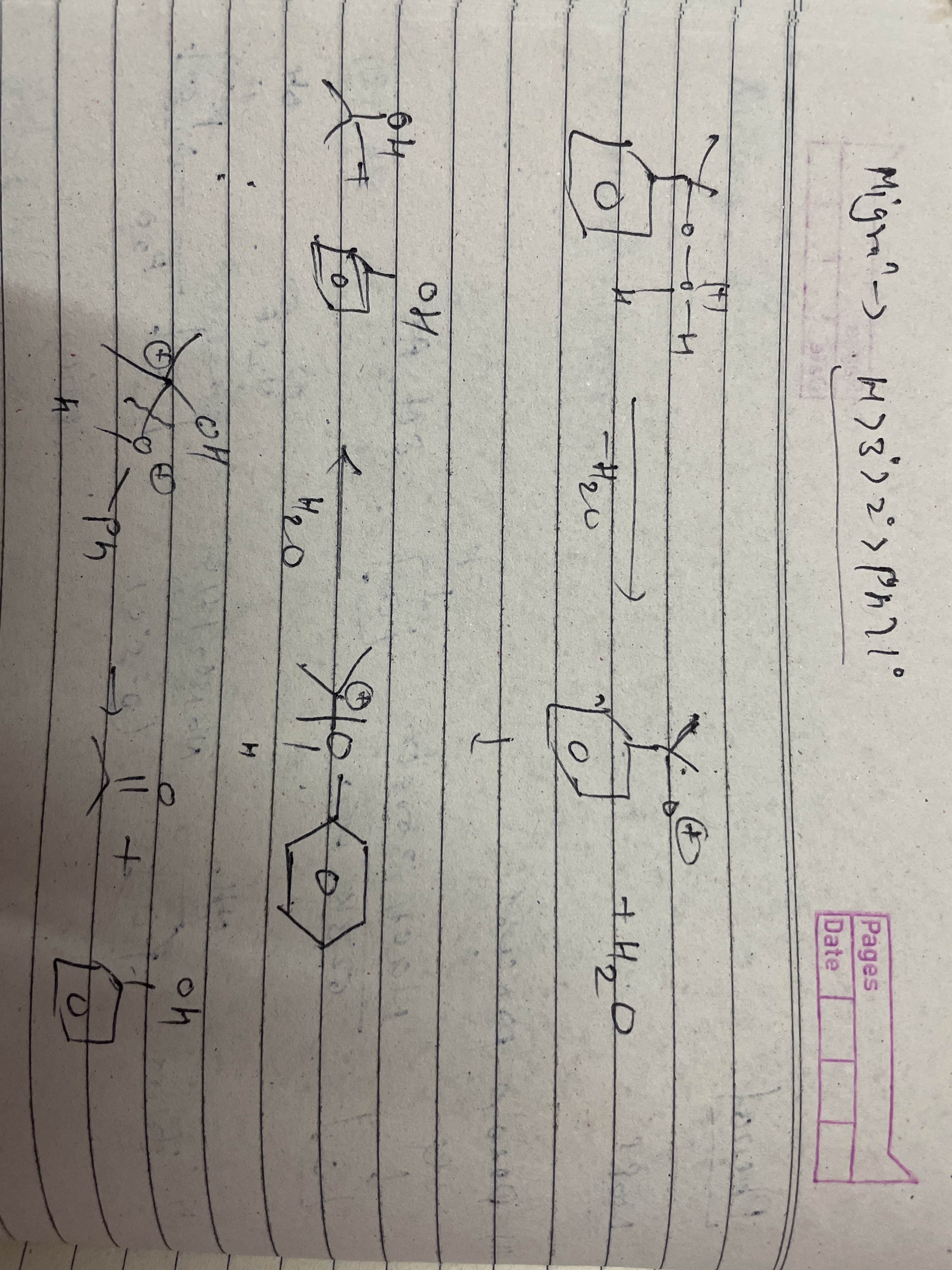
But i hope smj ayega apko
Yaar mech to dekha hai maine hnn par ye wlaa chiz ho kyu rha hai ye mera question hai
vo to aa gya dont worry there
konsa part?
Phele to H+ ka attack hua so wo right wle O pr lag gya waha se H2O nikal gaya
Ye uske aage ki rxn hai wo wla part piche wle page p tha
ye part
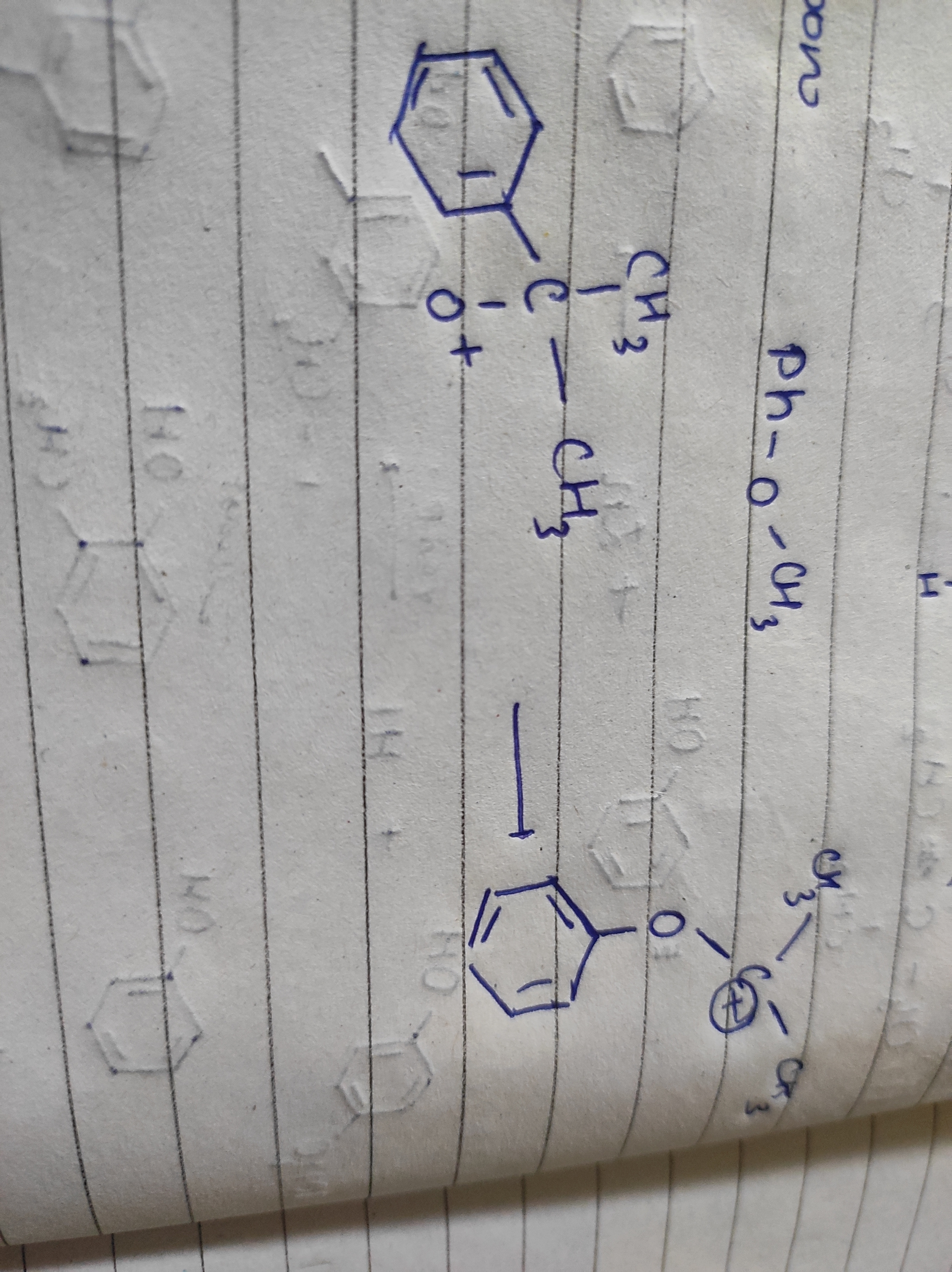
ye to hn good leaving grp ke karan ho jana
Us oxygen p ek hydrogen bhi hoga na
Apke wle me compound me ek H missing hai
hn sorry miss ho gya
hm fir waha Wo hydrogen oxygen pr shift ho jyega and acetone bahar nikl jyega
Phenol form hogya
vo to baad ki baat hai
ye shift kyu hoga?
ig waha pr zyada stablity hai
Oxygen ka lp bhi hai side me and 2 Methyl group
Aise bhi soch skti ho app ki o+ is very unstable
To ye wala shift krke hamne O ko bhi stable krdia and relatively + ki bhi stability aayi
hn vo to hoga
but benzene se koi bhi bond tod ke banan to tough haaina
ye oxygen h ke saath next step me bond hoga mai kabse sochri oxygen bond ho gya tab to +ve charge lelega ba
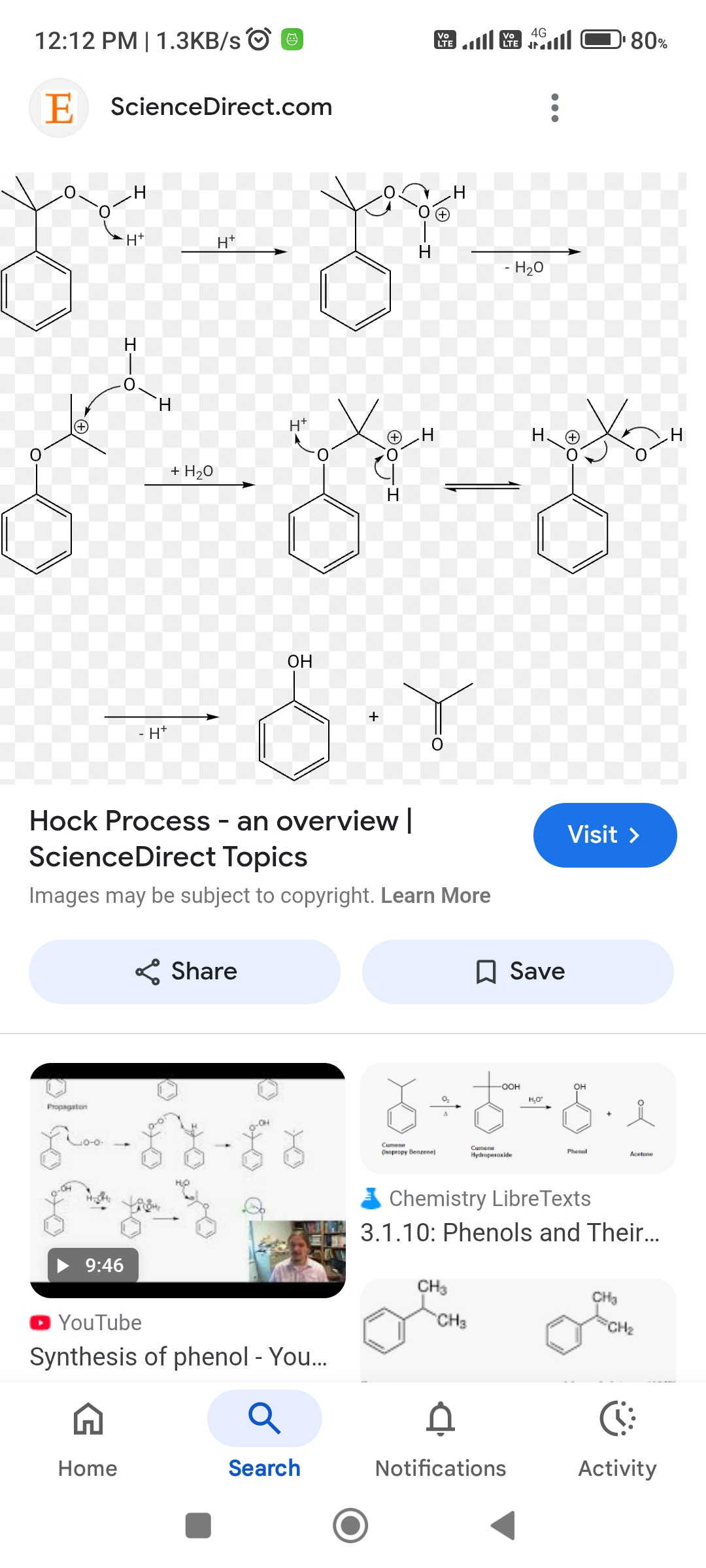
Ab isme third compound ko dekho
Kuch similar lag raha hai carbocation rearrangement se (1,2 shifting)
Usme ham kya karte the, ki ham ek group ko transfer karte the na more preferable was hydrogen right
Aur hamari ma'am ne hame bataya ki jab 1,2 shifting karte hai to hydrogen ke baad phenyl fir alkyl shift hota hai
To yaha bhi same chiz ho rahi hai
1,2 shifting se phenyl vaha par shift ho raha hai
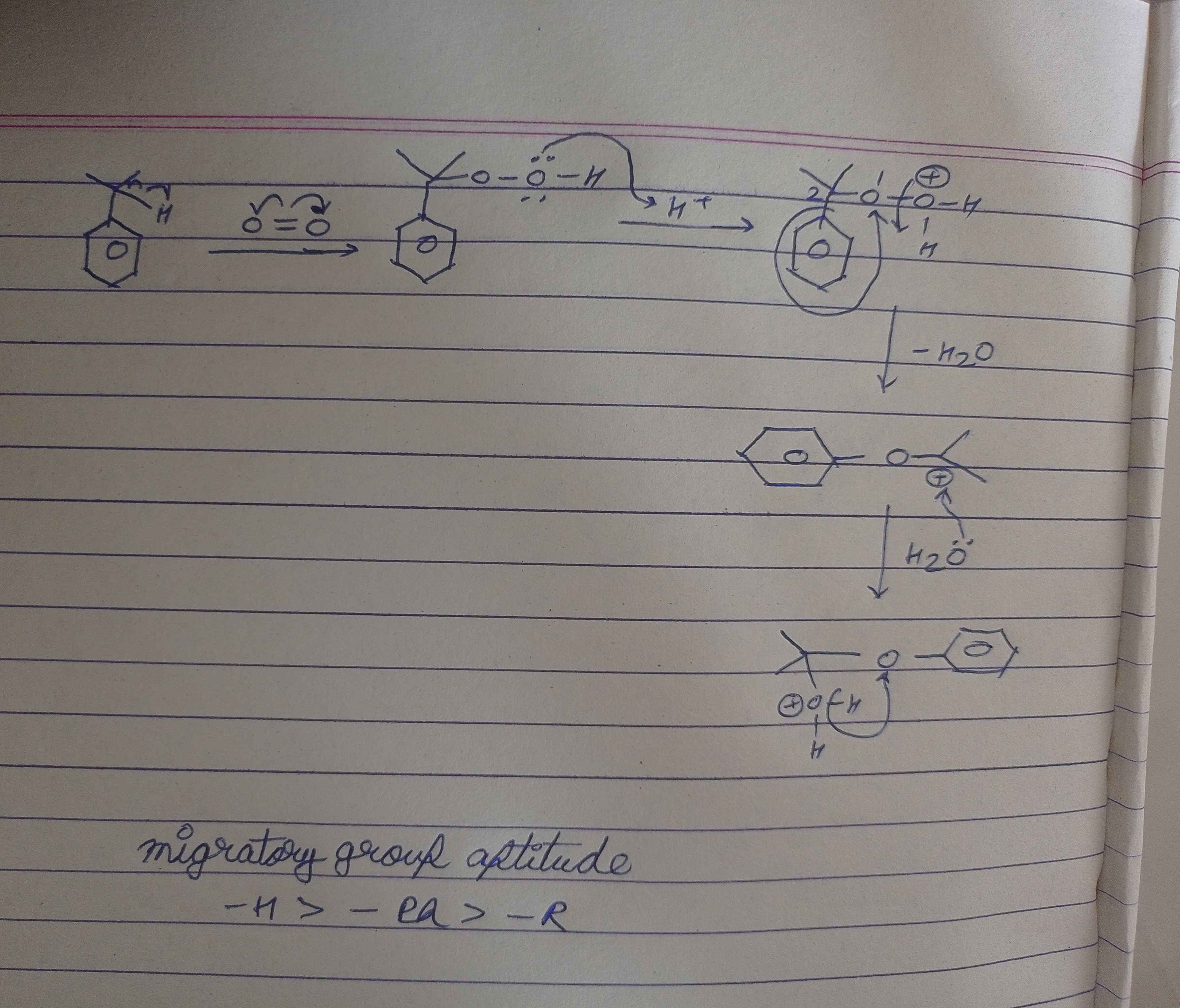
par ye jyda preferable kyu hai? Mtlb ye krne ke liye to benzene ke sath bond tut jayega aur vapis banega right? But vo thoda benzene kum hi psnd karta hai
I guess kyunki shifting ke baad oxygen ka lone pair benzene ke saath resonance mei participate kar raha hai ??
I don't know the actual reason but maybe...
Hamne direct benzene se juda bond ni toda na hamne oxygen or Me ke beecj ka bond break kia?
are mai kuch aur chiz bolri vaha
maybe could be
mai ispe thoda read karti
+solved @Prachi @Shreyaaa~
Post locked and archived successfully!
Archived by
<@888280831863451688> (888280831863451688)
Time
<t:1754915879:R>
Solved by
<@926887811674673172> (926887811674673172), <@1403315966720479295> (1403315966720479295)