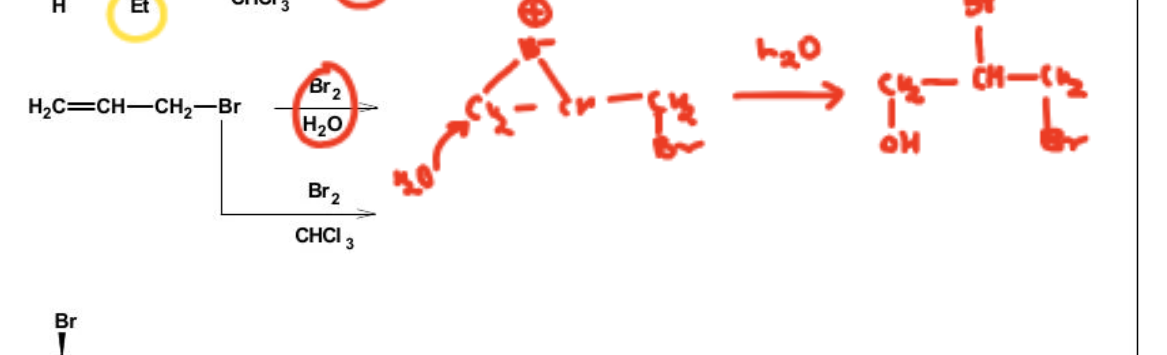OMDM doubt hydrocarbons alkenes (continued)
I had posted a similar doubt a few weeks ago, but I couldn't find the relevant problems where I was getting stuck. I have it better framed now, so please help me out with this. It has been bugging me for the longest time.
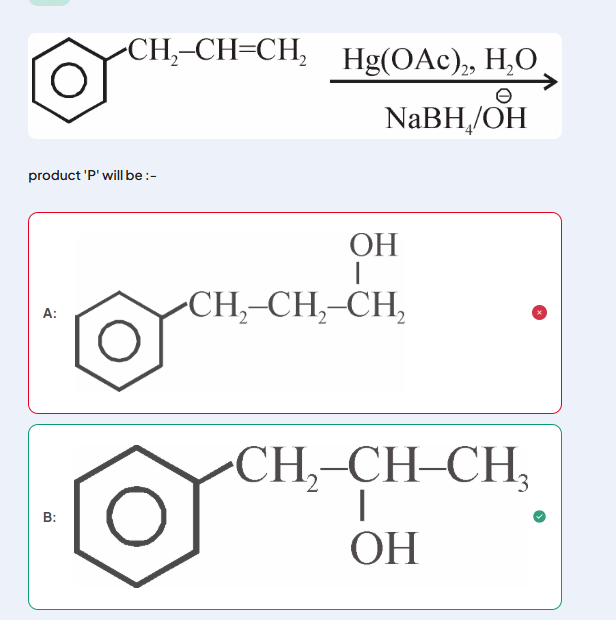
1. 1st image. I opened the cyclic mercurinium ion from the more stable cationic character which I thought to be at the other site due to -I effect of Ph. I thought +R won't be considered since they're not conjugated. But I was wrong.
2. 2nd image. Here we don't look at the +M effect of Br. Since it exhibits -I effect, we open the ring from the other site, for more cationic character stability.
If hyperconjugation were the reason, still what we did in the 2nd case can't be explained.
3. Now what if in a similar condition, we have a system of 2 non conjugated double bonds and 2 conjugated double bonds. What would be the products in each case? This will help clear a few things up for me.
1. 1st image. I opened the cyclic mercurinium ion from the more stable cationic character which I thought to be at the other site due to -I effect of Ph. I thought +R won't be considered since they're not conjugated. But I was wrong.
2. 2nd image. Here we don't look at the +M effect of Br. Since it exhibits -I effect, we open the ring from the other site, for more cationic character stability.
If hyperconjugation were the reason, still what we did in the 2nd case can't be explained.
3. Now what if in a similar condition, we have a system of 2 non conjugated double bonds and 2 conjugated double bonds. What would be the products in each case? This will help clear a few things up for me.