31 Replies
it wont right?
@Dexter
Note for OP
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.Yup
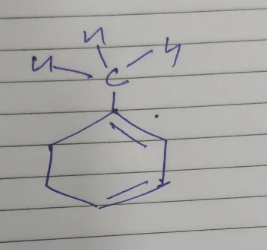
You got an sp3 carbon adjacent to a double bond
That has a hydrogen
Why not?
but it isn't in conjugation with respect to benzene right?
but it isn't in conjugation with respect to benzene right?
How does that prevent that hydrogen to be a part of hyperconjugation?
That double bond is still there right?
hyperconjugation ka definition use karo
Although delocalised but a fair share of double bond is still there
what does it say
.
.
It’s not benzene btw
Yeah
Didn't point out.....
yeah its cyclohex-1,3-diene
ismein 2nd point ka kya matlab hai fir, what am i getting wrong here

Given the slightest chance though, it would become benzene though
yaar ye sab mat use karo
use the defination to understand the concept
ye sab in the end kahi na kahi exception deke confuse kar denge
Usme ye likha hai bas ki side me double bond, positive charge, ya radical hona chahiye
Nahi sahi likha hai usme dw
Won't cause exceptions of any sort
Usne interpret galat kiya hai ise
well its oc and most of the time kaand hota hai
Are maine padha yaar
It's correct dw 😭
go over the definition it is easy, presence of partially filled p orbital or a pi bond
Extended likhna is wrong though
next to sp3c
Yeah better way to state the same thing
But can't call him wrong bas
That's why I mentioned
not wrong just it confuses
got it
thank you
especially when you learn for first time
It's fine
Hmm fair enough
Better to generalise
(at least in such cases)
sephrina
if ok then do close it if further doubt ask it for sure
ys?
+solved @Enamine @iTeachChem @Sephrina
Post locked and archived successfully!
Archived by
<@1380787313122213899> (1380787313122213899)
Time
<t:1755186474:R>
Solved by
<@984016629119713290> (984016629119713290), <@1035556259417571408> (1035556259417571408), <@888280831863451688> (888280831863451688)