FLOT
Should delta H-delta U be equal to -delta W which means it is P delta V which is P*(mass of 1 mol Sn)(1/density1-1/density2)?

14 Replies
@Dexter
Note for OP
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.$-10\cdot199\left(\frac{1}{5.73}-\frac{1}{7.31}\right)\cdot(10^{5} \cdot 10^{-3})$
This is what I'm getting but I'm messing up the powers of 10 somehow :/
SirLancelotDuLac
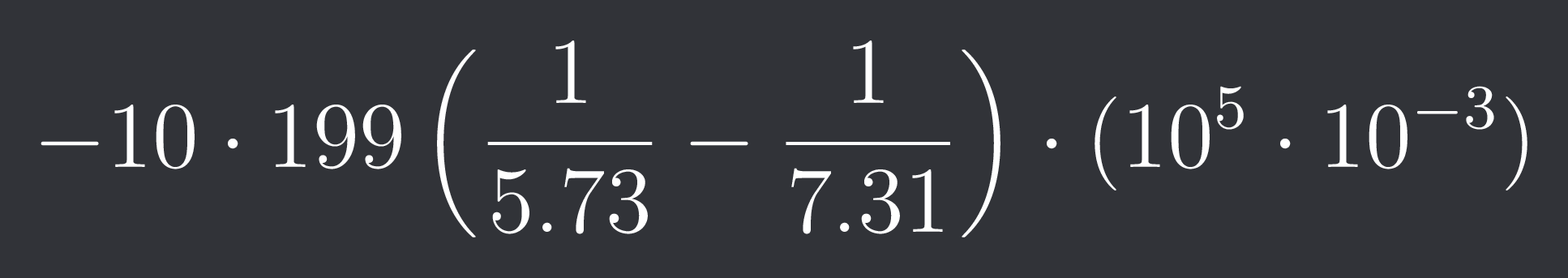
Lmao flot
You figured this out na
Nope, the powers of 10 are going wrong :/
I converted bar to Pa and g/cm^3 to kg/m^3
FLOT?:uhh:
First Law of Thermodynamics
You sure it’s not rt ln v2/v1 ?
Ignore that लोल
Isn’t the mass 118.71?


Do the calc in grams. You get volume in cc
Then convert cc to m3 and multiply pressure in Pa :) you get 4.4
4.5 if you round it off
Ohhh, I did kg to g 2 times 🤦♂️
Thanks a lot sirji 🛐
+solved @iTeachChem
Post locked and archived successfully!
Archived by
<@1075951732460376214> (1075951732460376214)
Time
<t:1756863225:R>
Solved by
<@1035556259417571408> (1035556259417571408)