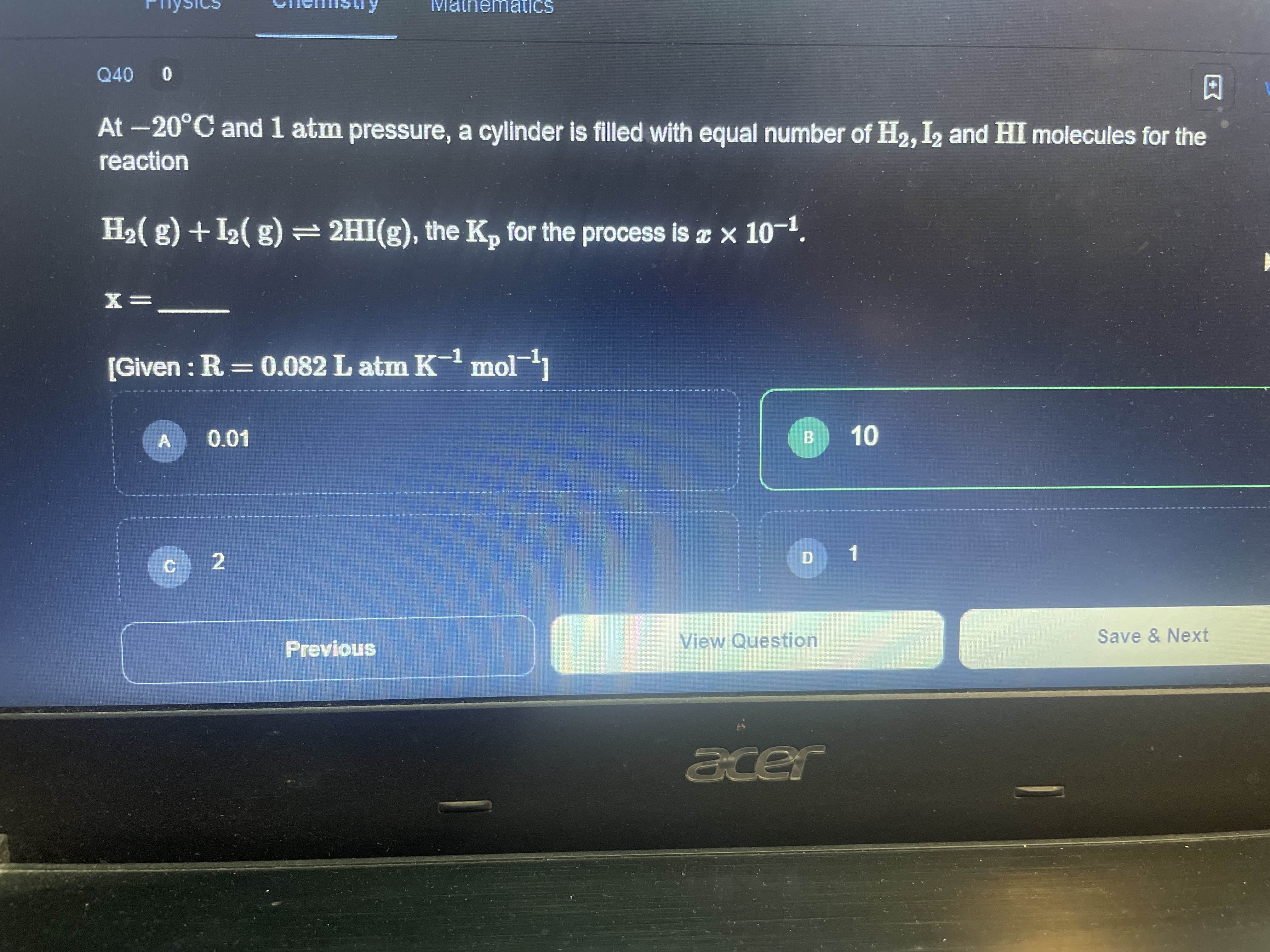
Eqbm
Is this que incomplete? Like they have given that initially number of molecules are equal..
But at eqbm the composition will be different they havent given any other think to calculate eqbm composition
But at eqbm the composition will be different they havent given any other think to calculate eqbm composition