12 Replies
@Dexter
Note for OP
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.@Prachi @Varun_Arora
i need help with 1 3 4
3rd is positive deviation. so B
4th is henry's law. first calculate the partial pressure of N2 in air
by using its mol fractn in air that has been provided
and then yeah write the exp for mol fractn in liq for n2. should give opt a
and for 1, idk if u can individually compare ya yb, xa xb with only what has been provided in the q
Why is there any deviation at all. Both are non polar. No h bonding or anything
I don't get this one
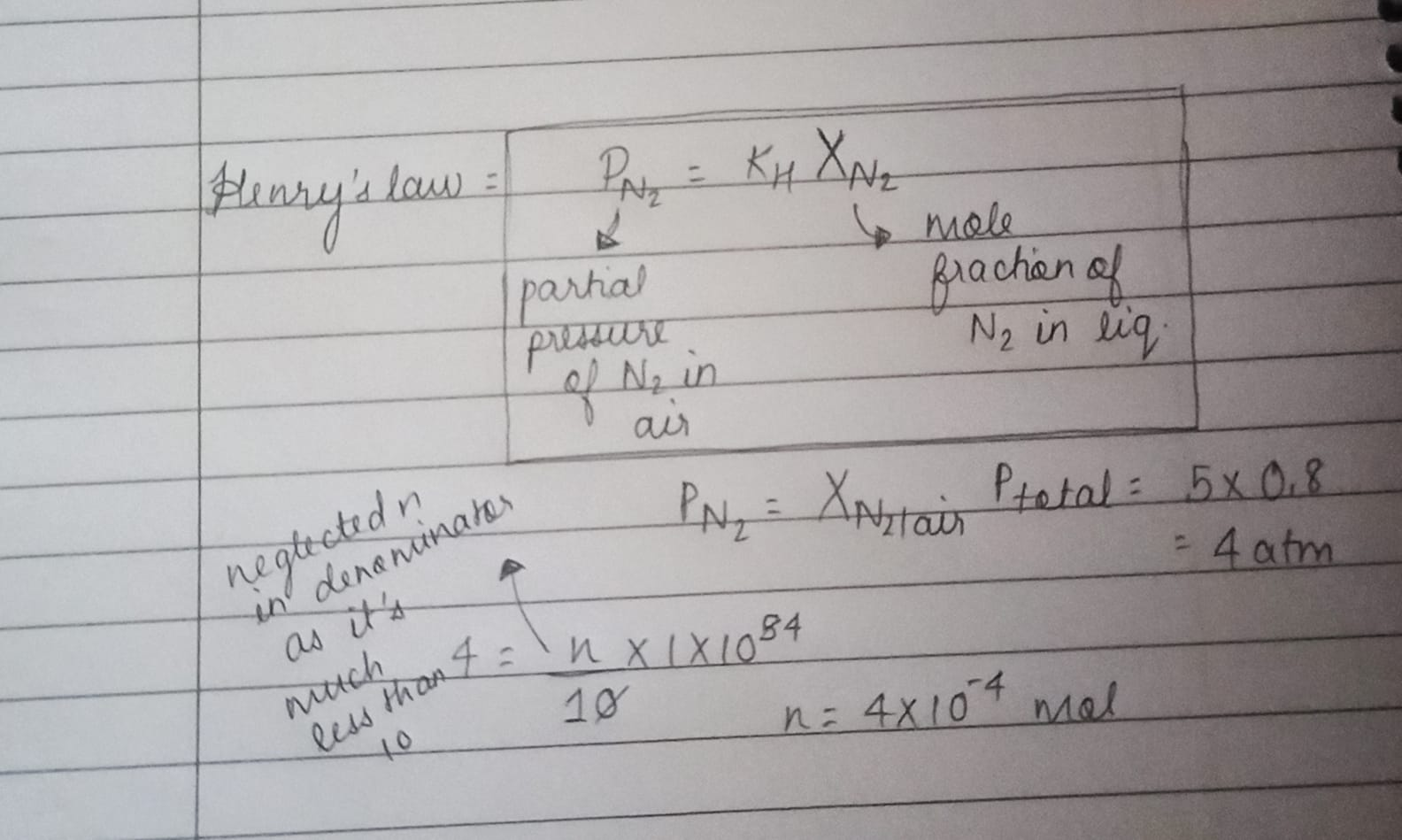
yeah true but the deviation depends on the relative magntiude right.. like the dispersion forces bw ccl4 and toluene will be weaker than those bw ccl4-ccl4 and toluene-toluene
plus toluene is aromatic
maybe this applies then? idk
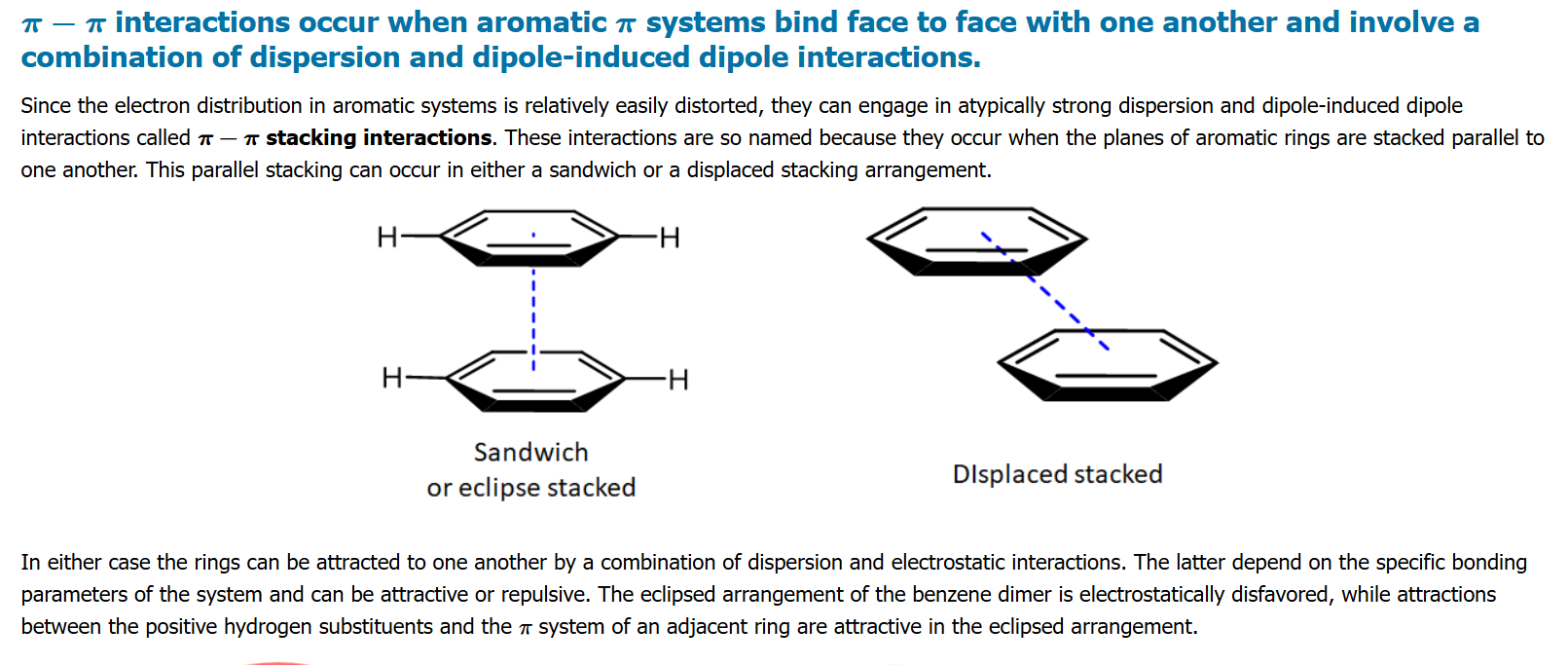
wont the forces be pretty much eqvt
very less polarity. very less dipole moment
hmm i feel it shouldnt but idk
@Varun_Arora
Yeah so....
I was busy somewhere
I'll see this room pe jaake
Pi stacking yup
CCl4 and Toluene decreases this
I mean mixing them
