Isomerism doubt
How to solve the first ques what should be the thought process got even more confused so made a separate doubt post. Please if someone can provide a detailed solution
47 Replies
@Dexter
Note for OP
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.first question of the ones marked for homework or like question number 1?? because the answer to question number 1 is there already...
number 1
I didn't understand it
even with answer
mainly trouble in knowing the more acidic H
Also how to write it's mechanism in acid and base
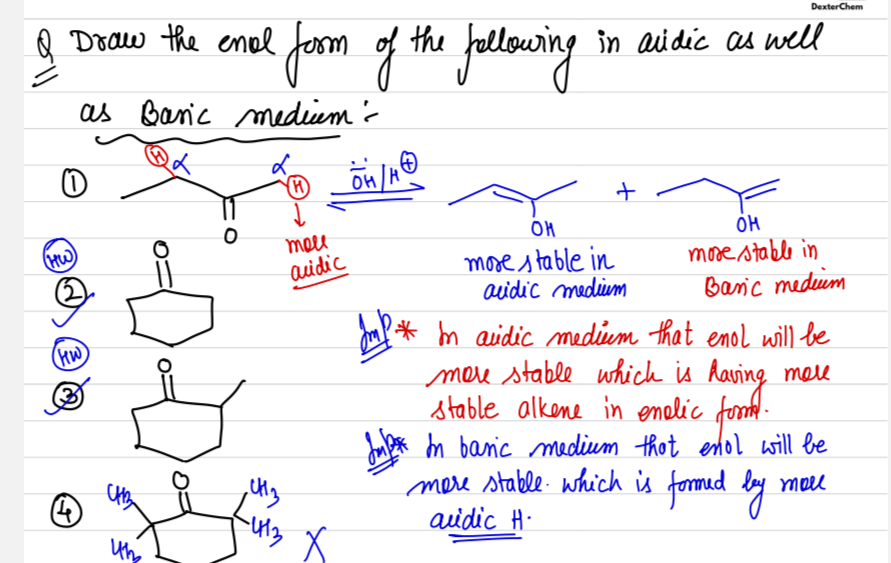
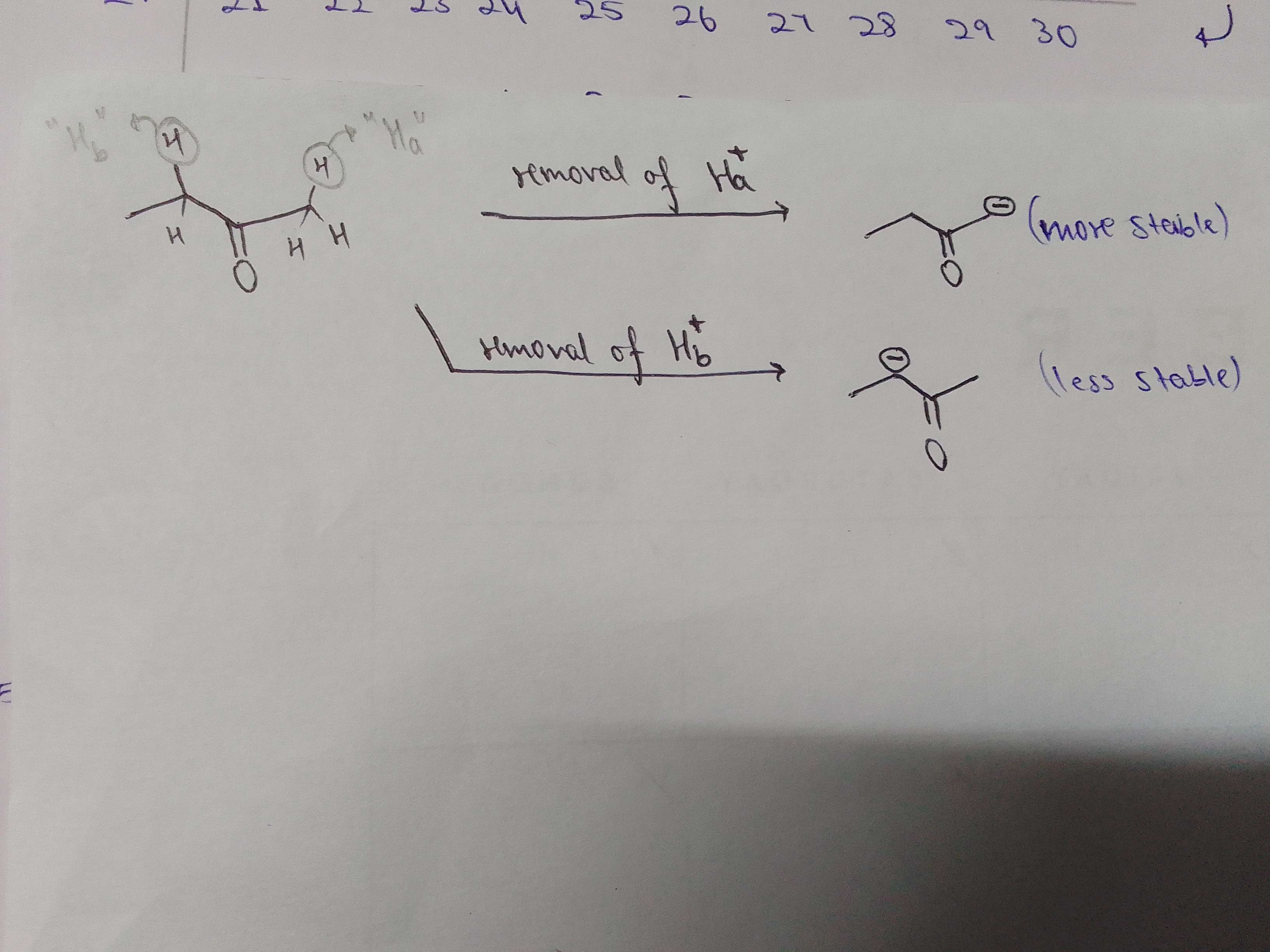
the conjugate base when you remove H(a) is more stable than when you remove H(b), which you can check thru stability of carbanion. You have a +I effect on the carbanion in the second case due to alkyl group
since removing H(a) gives a more stable conjugate base, H(a) must be a more acidic hydrogen, so in basic medium, the enol will be formed by the proton shift of the more acidic hydrogen, as base attacks acid
check stability of conjugate base
Second case means carbanion form by removal of H(b) ?
yes yes
Is this about aldol condensation?
tautomerism
basic medium mechanism
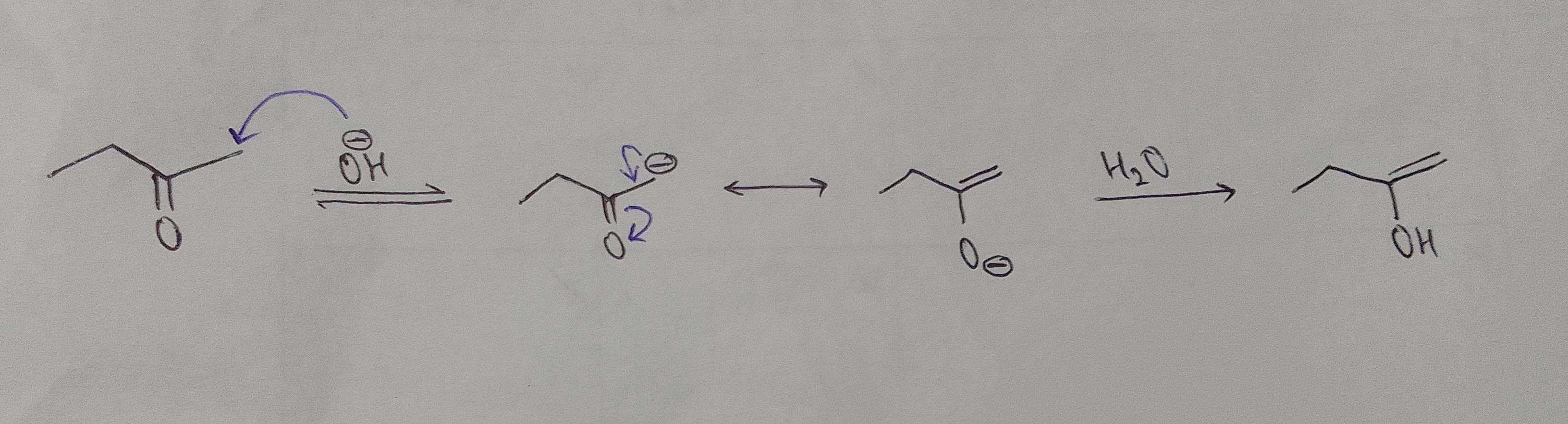
Keto to enol
Keto enol triad
Hnn usme me aldol condensation ki mech me hai ye vaha padhte hai
So in this how do I know OH- will attack H(a) only ?
Vaise vo ch3 grp hai
I mean H(a) is one of the hydrogen of that CH3 group
Right ?
Hn
Par vo attach to carbon pe karta hai
Ah yes
What I meant is how will ik OH- will attack on alpha carbon of H(a) ?
nono i labelled H(a) as the one on the right.. ig the pencil isn't that visible
And not alpha carbon of H(b) ?
H(a) is one of the hydrogen of CH3 only na ?
Chain Kam lambi pe karega cuz well carbonyl grp hai bagal me aur sath me hai h hai instead of ch3
Cause H(b) in ch2 ?
because H(a) is a more acidic Hydrogen than H(b)
yes yes and H(b) is the one on the left which is of CH2
And I will figure out that by finding stability of conjugate base ?
yes.
So basically you have to know the reaction and it's products before doing mechanism
It cant be the reverse
OH- will abstract the most acidic Hydrogen.
You will find the most acidic Hydrogen by comparing the conjugate bases of all the available alpha carbon's acidic hydrogen
kinda
Look at step 1 that's how enolate is formed usi tarike ka mech yaha lagega

Can we say h(a) is more acidic cause the carbanion formed by its alpha carbon has 2 alpha h ?
While h(b) alpha carbon carbanione has 1 alpha H ?
nono, you need to compare stability of that carbanion by checking inductive and mesomeric effects
Ohk
yee but here there is only one site for attack, but we're dealing with multiple sites
Thats the general idea and you go to the site which is more susceptible to reaction aka less stable and the attack will be there, now that depends on the groups attched
Like Carbonyl groups will take away electrons and methyl will give away so based on which is more reactive that will get attacked
Simple
all this made me realised my concepts related to inductive effect are not strong
Bhai itna naam kyu Dena hai terko ?
Sidha kya hora hai electron ke naam pe vo smj na
Confuse to hoga hi agar 50 naam ke hisab se yaad karoge
like how guide me
Just learn ki konse grp electron Lena psnd karte hai konse dena
Kaha par electron kaise jayega
Uska flow kaise hai vo smjho
Are ek badhia goc revise krke 30-40 pyq laga de sara clear ho jayega confusion
True 2024 2025 ke goc pyq kar leta hu
Kal hi allen ne booklet bhejo hai pyqs waali chapterwise
btw acid are electron acceptor so acid always exert -I effect right @Sephrina
Yss
looks like I am finally getting the flow of GOC and stuff
Yep keep doing it also, close the doubt if you ok ,ping me in gen or dm if you got any other ques
+solved @Sephrina @silverdew
Post locked and archived successfully!
Archived by
<@754965160803696670> (754965160803696670)
Time
<t:1759947899:R>
Solved by
<@888280831863451688> (888280831863451688), <@780664223491162112> (780664223491162112)