Ionic Mobility
This is a jee mains pyq but I don't understand the reasoning given if it's even true or not, the answer given is 1
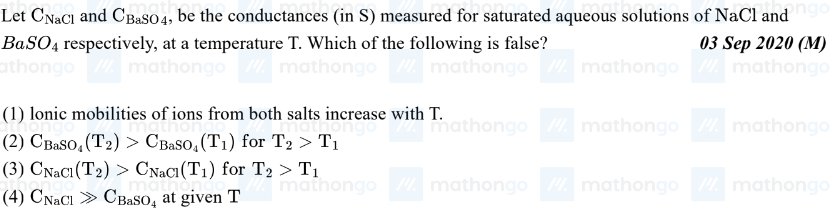
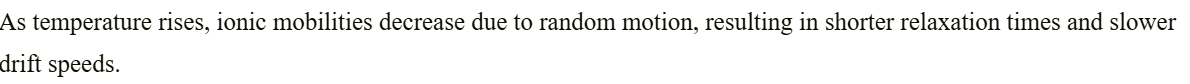
12 Replies
@Dexter
@Dexter
Note for OP
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.Note for OP
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.This is in Zumdahl, check out drift velocity and relaxation time between collisions of any random gas particles. It is in the chapter that has the derivation of kinetic theory of gases.
Same idea applies here.
LOL back then they would pick things directly from books that you may not have read :P
but when temp inc the viscosity dec for liquids , and by stoke's law, mobility is inv prop to viscosity so it has to increase right
i found this too
https://chemistry.stackexchange.com/questions/165536/variation-of-conductance-of-saturated-aqueous-solution-with-temperature
Chemistry Stack Exchange
Variation of conductance of saturated aqueous solution with tempera...
The following question was asked in JEE Mains 2020:
Let $C{\ce{NaCl}}$ and $C{\ce{BaSO4}}$ be the conductances (in S) measured for saturated aqueous solutions of $\ce{NaCl}$ and $\ce{BaSO4}$,
couldnt find zumdahl pdf but in some yt videos it didnt mention ionic mobility
also these are aqueous solutions right, we can apply ideal gas laws here??
Yea cos we do this in infinite dilution.
So not directly but ktg kinda applies
Is what I recall
Oh can you tell me some examples? I can't recall properly
Looks like this has different opinions
https://pubs.acs.org/doi/10.1021/ja00198a007
When I get home will check book.
i see but again this is for gases like he, co2 and sf6 and not aqueous electrolytes so is it right to extrapolate to aqueous medium?