21 Replies
@Dexter
Note for OP
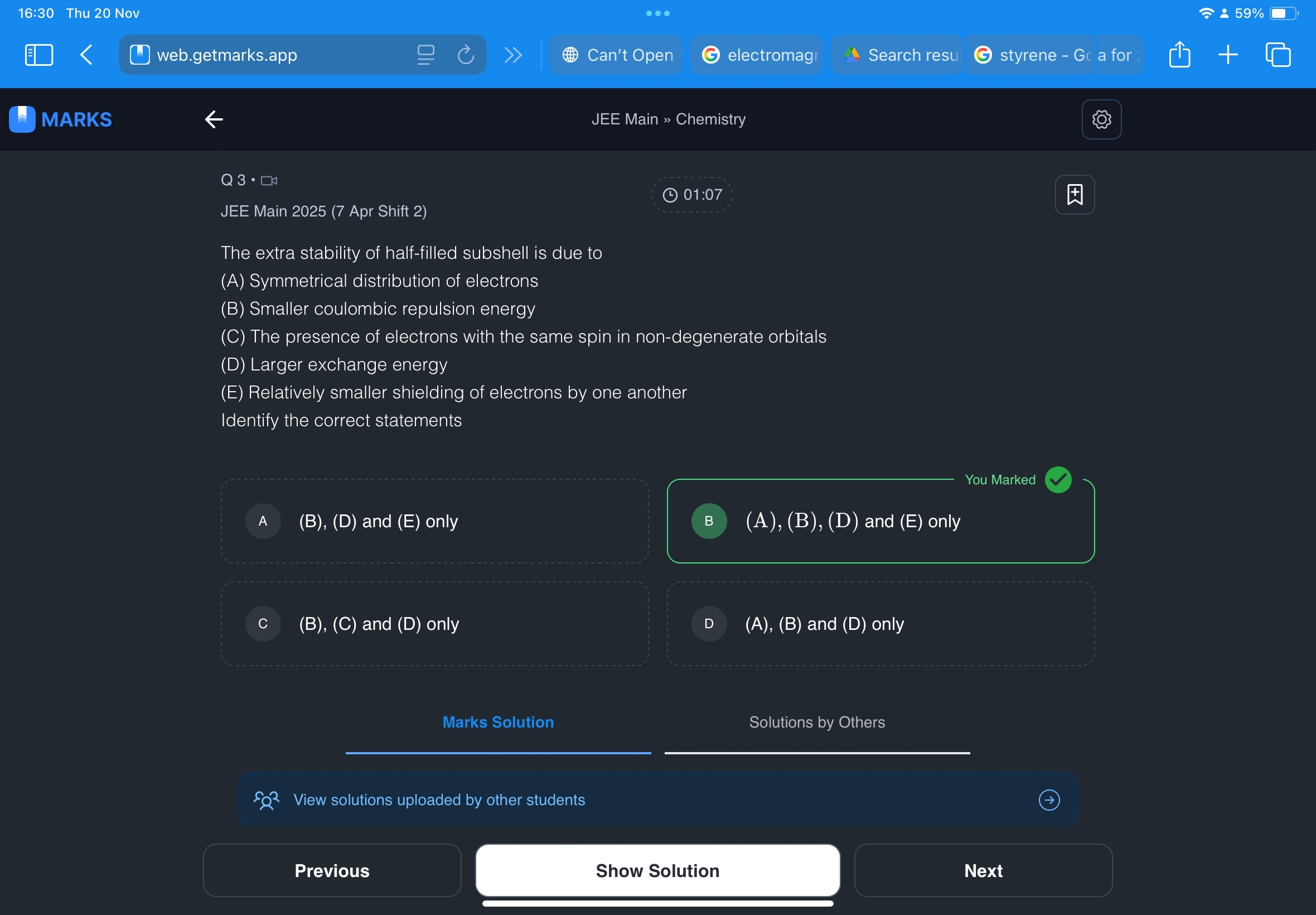
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.exchange energy hai
what is this symmetrical lol
unless they mean same spin
- symmetry equals least repulsions possible hence good
- smaller repulsion means more stable
- large exchange energy means we need to supply a lot of energy to do something ( change the config ) which means it is stable otherwise it would do such easily
- less shielding means less repulsions by other electrons means more stability
That is point D
And as for C option
It's every orbital me same number with same spin afaik
got it
How symmetry is related to repulsion though
No degenerate means the orbitals with different energies, and well we know that if same spin are present together it is not good
Non degenerate se farq hi nhi padega na since half filled subshell ki baat ki hai
Because day for example I have 2 electrons really close and the third one far away then the two will have a lot of repulsion and the third will have less which was created chaos
Exactly
Suppose you have a 2p subshell usme 3 orbital hai
Now you've got 3 electrons in 2p
Oky
as per hund rule pehle singly hi bharenge
Ab suppose kr
Right
Agar wo jo 3 electrons hai
Sabke same spin hai
And teeno orbital me ek ek electron hai
Yeah
To they have ample amount of space bw them
Ab same spin hai to sab repel kr rhe
And since they are symmetrically distributed all the forces that act
More or less
Becomes constanr
Hence giving them stability
Ahh i see
Smj gye?