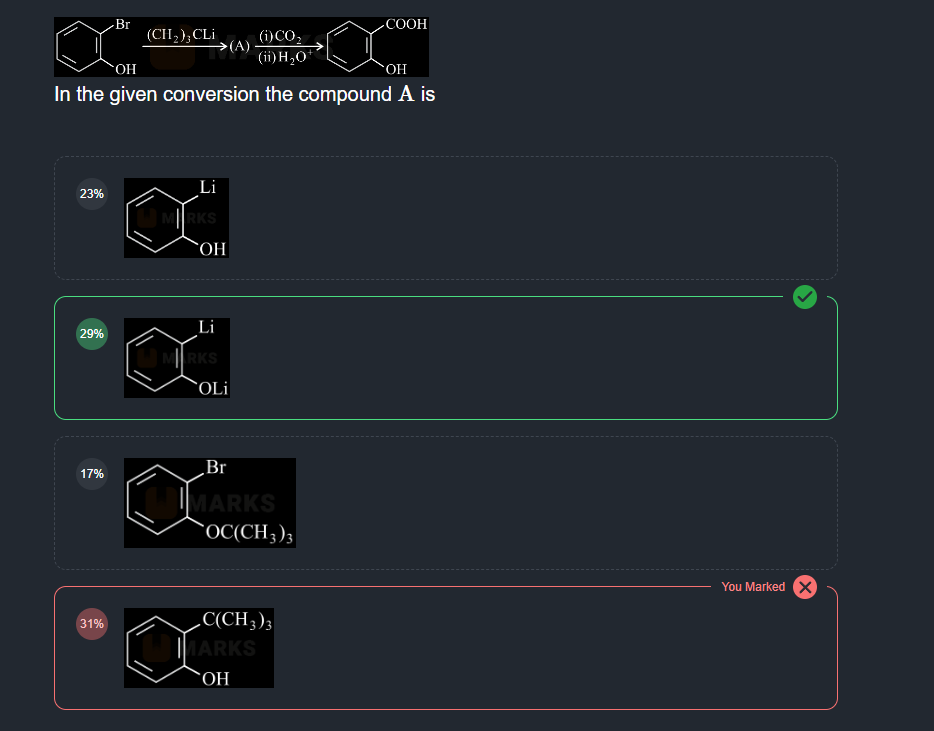
9 Replies
can someone share the mechanism pls
@Dexter
Note for OP
+solved @user1 @user2... to close the thread when your doubt is solved. Mention the users who helped you solve the doubt. This will be added to their stats.isnt the ring rich in electrons?
so only electrophiles would attack it?
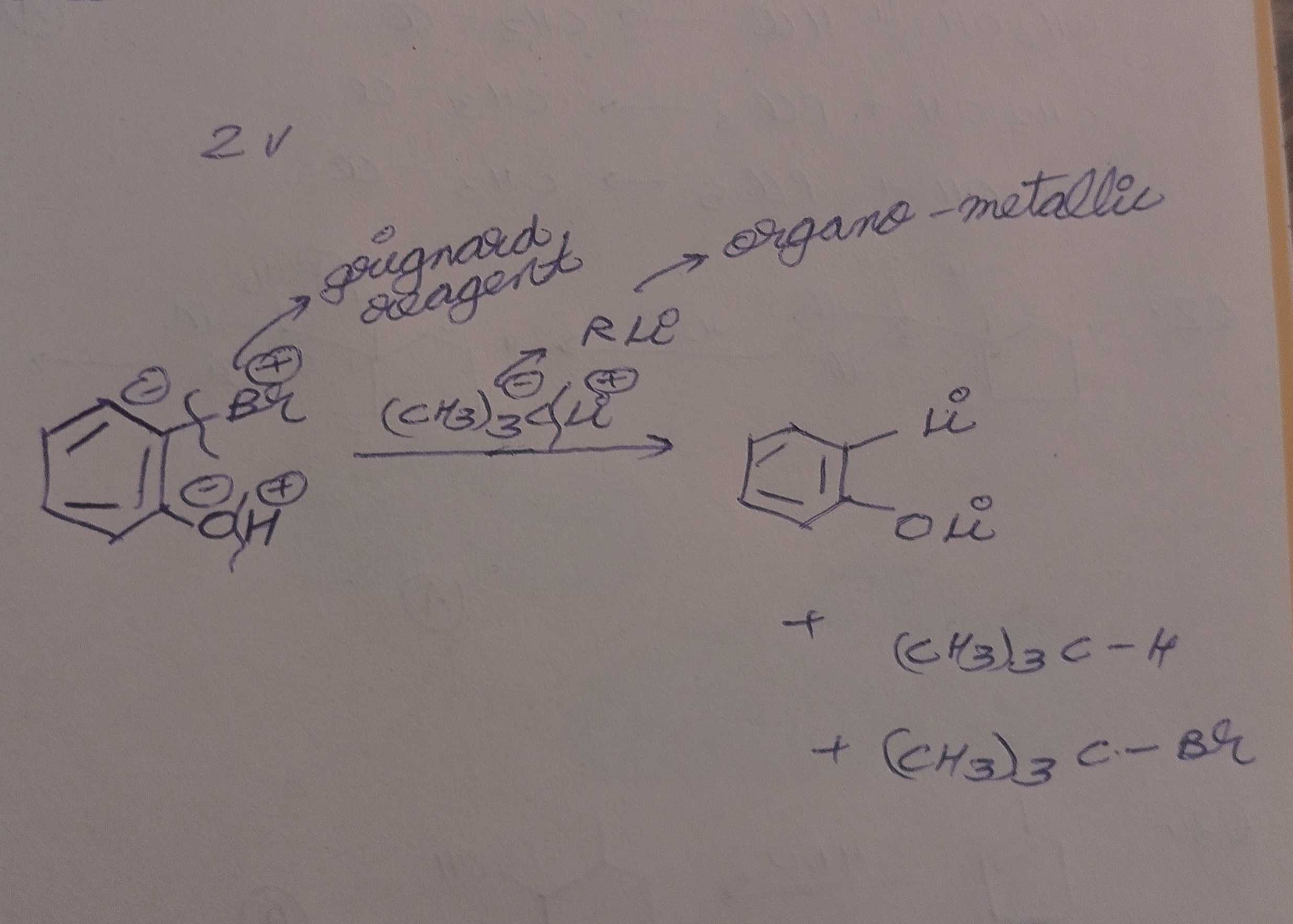
why did the br bond break tho
is it something we should learn?
Oh, shitt
Forgot about this
Right..
So, I asked my teacher about this and she said this happens rarely but it is known as halide exchange reaction
The C(CH3)3- ion will attack on Br, and as we know Br is a good leaving group, so it goes with C(CH3)3- to form C(CH3)3Br leaving a negative charge on the benzene ring with reacts with Li+ ion
So, what I did above with Br is wrong, but with OH it's correct
ohh oke
got it thanks
+solved @Prachi
Post locked and archived successfully!
Archived by
<@1336740240563372134> (1336740240563372134)
Time
<t:1764679546:R>
Solved by
<@926887811674673172> (926887811674673172)