Epoxide + grignard
Problem 7
I first opened the epoxide ring from c=o wala carbon and then did reduction of the giving propan 1,2 diol
giving propan 1,2 diol
I first opened the epoxide ring from c=o wala carbon and then did reduction of the
 giving propan 1,2 diol
giving propan 1,2 diol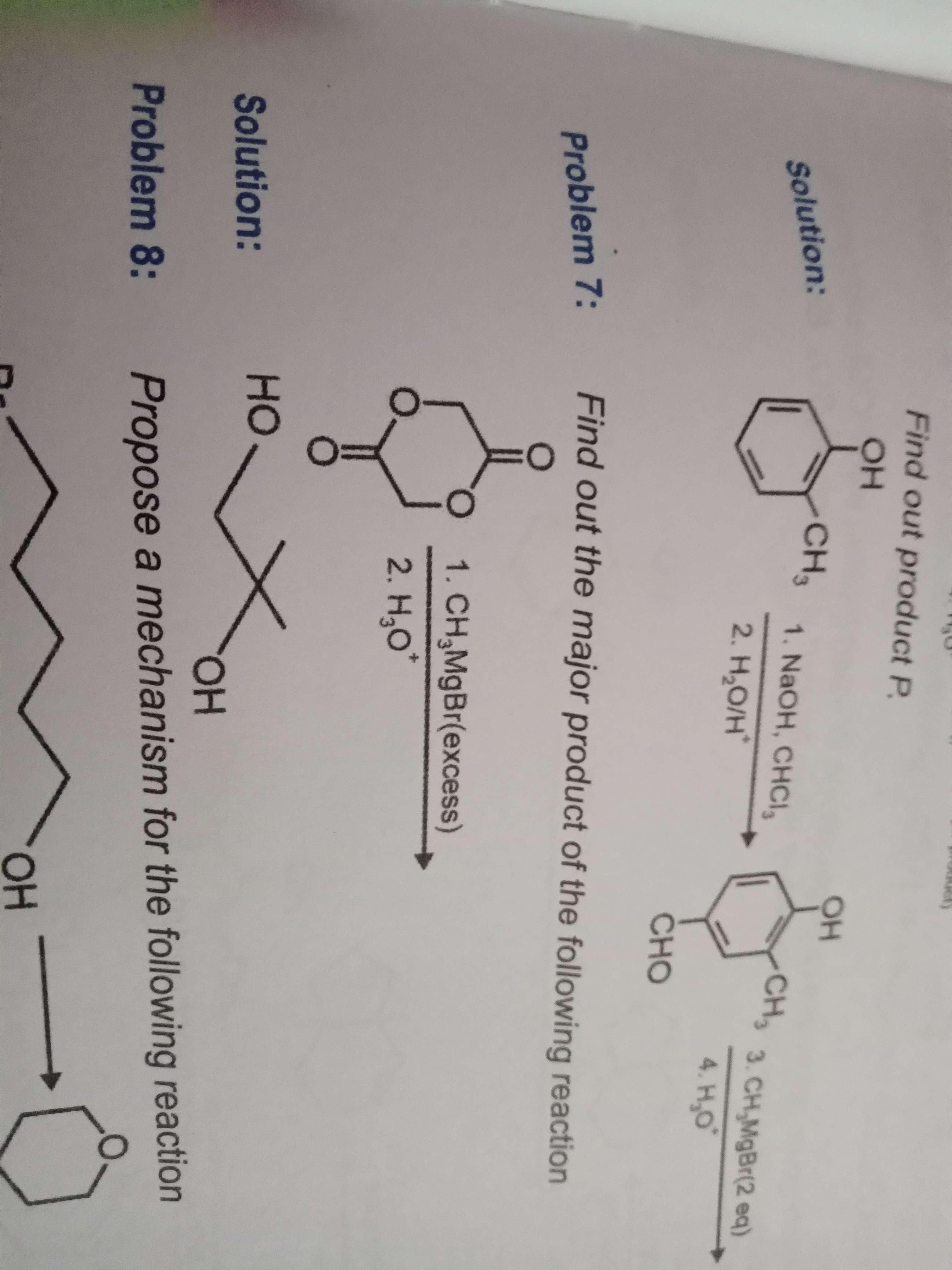
 giving propan 1,2 diol
giving propan 1,2 diol