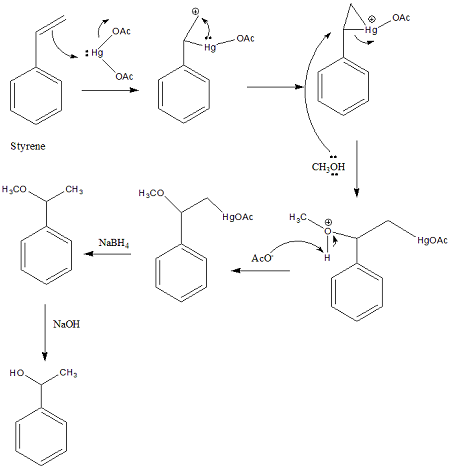
omdm reaction mechanism doubt
so I was solving this problem in omdm... In omdm a mercurinium ion (non classical) formation takes place. My teacher said that we've to break this from the site where there's more cationic character, determined by inductive and NOT mesomeric.
Then in the image I've attached, why do we break from the site where phenyl is attached. doesn't it exhibit -I effect? It is better cuz of resonance, but my teacher told we shouldnt look at that.
Then in the image I've attached, why do we break from the site where phenyl is attached. doesn't it exhibit -I effect? It is better cuz of resonance, but my teacher told we shouldnt look at that.