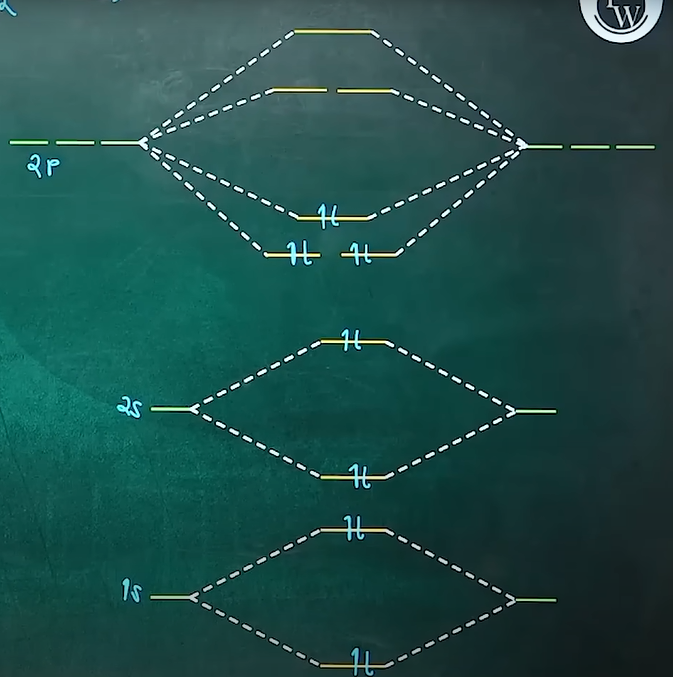
MOT
bruh, here we took N2 which has like 14e. so in MOT, for total e < 14, we consider or believe that pi 2px and pi 2py have lower energy than sigma 2pz so like my doubt is when we fill the electrons, we follow the hund's rule and aufbau principle, so like if a molecule has 10 electrons, as per what i have learnt is that when we start filling, we fill the lower energy orbitals first but since we have only 10 electrona, pi 2px and pi 2py will get occupied first so this means that pi bond is established before sigma, how?
or am i misinterpreting the ENTIRE MOT
or am i misinterpreting the ENTIRE MOT